Introduction
As one of the most common musculoskeletal conditions, low back pain (LBP) affects more than 80% of the global population and is a leading cause of lost work time, doctor visits, and decreased quality of life [1]. Approximately 10% to 40% of patients with LBP progress to develop chronic LBP [2, 3]. Most cases of LBP fall under the category of non-specific LBP (NSLBP), which has no identifiable disease or underlying cause and affects 90% of the LBP population [4].
Weakness of the superficial trunk as well as abdominal muscles is an essential contributing factor to LBP. So, strengthening these muscles is usually linked with major reductions in LBP and lower functional impairment [5]. Patients with LBP have insufficient activation of the lumbar multifidus (LM), transversus abdominis (TrA), as well as obliquus internus (oi) muscles, all of which contribute to lumbar spinal stability [6].
NSLBP has a significant influence on proprioception [7] and the spatio-temporal parameters of gait [8, 9]. The findings can be used in selecting better rehabilitation procedures [8]. Walking patterns in elderly people with LBP are significantly distinct from those in older adults without LBP, even after controlling for age and gender. The wider the steps, the higher the risk of LBP when walking quickly [9].
Clinical practice guidelines suggest exercise therapy as an appropriate therapeutic option for those suffering from NSLBP. Patients with CLBP can benefit greatly from a program of segmental stabilization as well as strengthening exercises [10, 11]. Patients having LBP commonly suffer from a lack of hip abductor strength [12]. Muscle strengthening exercise, which may involve lumbar exercises, is a great addition to exercise treatment programs for individuals with LBP for the purpose of rehabilitation as well as preservation of easy daily living [13]. Core muscle dysfunction in NSLBP can contribute to poor motor control during gait which may lead to compensatory movement [14].
Although lumbar stabilizing exercises and progressive hip strengthening exercise programs were already tested for their effect in managing LBP, up to the researchers’ knowledge, there is a gap in the body of knowledge about their effectiveness on spatio-temporal gait parameters. So, the objective of the study was to evaluate the impact of progressive hip strengthening exercises when applied in combination with lumbar stabilizing exercises on pain, disability, and spatio-temporal gait parameters in patients with NSLBP.
Subjects and methods
This study was a randomized controlled trial. Patients were recruited from the physical therapy outpatient clinic of Cairo University. From december 2021 to december 2022, patients were evaluated in a physical therapy clinic where they received treatment.
Subjects
Eligibility was determined for all individuals with NSLBP who were referred for physiotherapy by a neurologist. Patients were included if they experienced pain below the level of T12 but not below the buttock line, were between the ages of 18 and 60, and had chronic nonspecific LBP (greater than 12 weeks). Cancer patients, those with spinal cord injuries or spinal osteoporosis, those with primary joint diseases including active rheumatoid arthritis (RA) or metabolic bone disorder, and those with significant psychological problems were not included.
Sample size
To reduce the probability of type ii errors, the sample size was calculated before the study began. Using an effect size of 0.41, a power analysis of 80%, and a two-tailed significance level of 5%, G*Power (version 3.1.9.2; Germany) determined the necessary sample size for the study. it was originally expected that there would be 49 patients with NSLBP, but after considering dropouts, the overall sample size was calculated to be 60.
Randomization
Sixty people with NSLBP were randomized between two groups (A and B). A random block generation computer tool, found at http:/www.randomization.com/, was used to assign groups at random. To reduce the potential for bias and variability among the two groups, patients were randomly assigned to blocks of four, six, and eight with an allocation ratio of 1:1. The author, who had no role in participant selection, data collection, or treatment, used opaque, sealed envelopes to make the concealed allocation. Another author applied baseline and 6-week measurements. Finally, the envelopes were opened by an author who continued with the treatment consistent with group allocation.
Outcome measures
All outcome measures were evaluated at baseline and 6 weeks later for each treatment intervention.
Pain intensity level: The pain intensity was measured utilizing a Visual Analog Scale (VAS). This measurement tool uses a transverse continuous line of 100 mm in length, beginning with no pain at all on the left side while the right side represents the worst pain imaginable. The patient is requested to express their degree of pain according to the scale. The VAS is generally regarded as a valid and reliable tool for pain measurement [15, 16].
Disability assessment: The Arabic version of the modified oswestry disability Questionnaire (ModQ) was used. This index is a well-validated, self-reported questionnaire created for LBP with ten components in the Arabic version [17]. it is a valid and reliable tool for the assessment of patient function [18]. There are 10 questions on this self-evaluation questionnaire, and each one can be graded from 0 to 5. These include pain, self-care, lifting, moving, walking, sitting, standing, and sexual and social life, as well as travel and sleeping difficulties due to LBP. The total score is divided by 50 and then multiplied by 100, giving a resulting percentage of disability ranging from 0% (no disability) to 100% (total disability). Scores on this scale can be interpreted in the following ways: Below 20% impairment is considered minimal disability, between 20% and 40% is considered moderate disability, between 40% and 60% is considered severe disability, between 60% and 80% is considered debilitating LBP, and above 80% is considered excessive incapacity (bed-ridden) [19].
Spatio-temporal assessment (Kinovea 2d free software, version 0.9.5): The spatio-temporal gait parameters include stride length, stride time, stride speed, cadence, and CoG vertical displacement. Kinovea two-dimensional (2d) analyses have been shown to be a valid, reliable, accessible, low-cost, and free alternative to assess lower limb kinematics during gait [20, 21]. The goals and methods of the study were thoroughly discussed with each participant, and all instructions were given verbally. Reflective markers were applied to the anterior superior iliac spine, greater trochanter, lateral condyle of the femur, and lateral malleolus of the fibula [22]. despite the fact that the system (Sony Camera-Kinovea) utilized is technically a markerless motion capture system, the markers help Kinovea focus on the action when it’s time to track the action (play back the video) [23]. Each participant was instructed to walk at a normal pace along the 5-meter walking route. A high-speed digital video camera (Sony HXRNX5U NXCAM; Sony Corp., Minato, Tokyo, Japan) was used to record the gait. The camera was placed on a level tripod approximately 0.3048 m (1 ft) above the floor and at a distance of 2.43 m (8 ft) perpendicular to the center of the pathway [22, 23].
The walking protocol was performed 5 times, and each time a video was taken using a Sony camera to capture the action (with an emphasis on the walking gait). Marker placement and the remainder of the experiment took approximately 20 min in total for one individual [22]. Each recorded video was first transferred to a computer, where it was then played again to look for any evident problems. Furthermore, poor-quality captures were eliminated. For the accepted videos, Kinovea was used to find and identify all four major markers and to follow the movement of the markers during the whole clip (walking gait) [23].
To account for initiation and fatigue, the first and last cycles of gait were excluded from the study. Thus, only the intermediate (2–4) cycles were examined. Following the completion of the video tracking process, the software’s capabilities were used to measure and record the gait spatio-temporal characteristics (speed, cadence, stride time, stride length, step length, step time, and CoG vertical displacement) [20–23].
Intervention
Patients in each of the two groups performed the same set of exercises under the close supervision of a physical therapist. Each patient was scheduled for 3 sessions per week over a 6-week period. Each treatment visit lasted 30–45 min. All participants received an education session at the beginning.
Group A: control group
Patients received only lumbar stabilizing exercises. Each exercise required an isometric hold that lasted for 7–8 s. Ten repetitions of each exercise were performed, with 3 s of rest between sets. The patients rested for a full minute between each exercise [24, 25]. The description of lumbar stabilizing exercises is shown in Supplementary 1.
Group B: study group
Hip strengthening exercises
Patients received lumbar stabilization exercises in addition to progressive hip strengthening exercises. Progressive hip strengthening was done according to the delorme protocol. First, the 10-repetition maximum was determined for each patient. Then the therapist uses 50% of the 10-repetition maximum as the training load for the first 2 weeks, then 75% of the 10-repetition maximum in the second 2 weeks, then 100% of the 10-repetition maximum in the final 2 weeks. All exercises were performed 10 times per set for 2 sets per session. Three sessions per week for 6 weeks were prescribed. Each exercise was performed for 30 s at full isometric contraction, followed by 10 s of rest in the starting position [26]. The description of hip strengthening exercises is shown in Supplementary 2.
Statistical analysis
Shapiro–Wilk was used to evaluate for data normality. Levene’s test for homogeneity of variances was performed to examine the homogeneity among groups. The data followed a normal distribution, and the variances were the same. The subjects’ characteristics were compared between groups using an independent t-test. Analyses of treatment effects on VAS, Modi, CoG vertical displacement, stride length, stride time, stride speed, and cadence were performed using a mixed MANoVA. For further multiple comparisons, post-hoc tests with the Bonferroni correction were performed. All statistical tests were performed at the p < 0.05 level of significance. The Windows version of the Statistical Package for the Social Sciences (SPSS) version 25 was used for the analyses.
Results
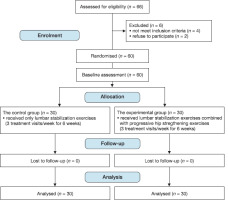
Baseline data as well as subject characteristics for both the control and study groups are presented in Table 1. Age, weight, height, and body mass index did not differ significantly (p > 0.05) between groups. in addition, baseline data did not vary significantly (p > 0.05) between the groups. The flowchart of the study was presented in Figure 1.
Table 1
Comparison of age, weight, height BMi, and occupation between control and study groups
A significant interaction between treatment and time was found using mixed MANoVA (F = 8.97, p = 0.001, 2 = 0.54). The time factor was found to have a significant main effect (F = 76.77, p 0.001, = 0.97). The treatment main effect was statistically significant (F = 2.82, p 0.01, 2 = 0.27). According to Table 2, there were no pre-treatment statistically significant differences among the groups. There was a significant decline in VAS, Modi, and CoG vertical displacement in the study group when compared to that of the control group post-treatment (p < 0.05). No significant differences were detected in stride length, stride time, stride speed, and cadence between control and study groups post-treatment (p > 0.05, Table 3).
Table 2
Baseline clinical characteristics of the subjects (n = 60)
Table 3
Between-group comparisons after 6 weeks of interventions (n = 60)
There was a significant decline in VAS, Modi, CoG vertical displacement, and stride time post-treatment compared to pre-treatment in the control and study groups (p > 0.05). There was a significant increase in stride length, stride speed, and cadence post-treatment compared to pre-treatment in the control and study groups (p > 0.01) as shown in Table 4.
Table 4
Within groups changes pre-post intervention of control and study groups
Discussion
The present study aimed to examine the impact of adding hip strengthening exercises to the lumbar stabilization program on pain, disability, and the spatio-temporal parameters of gait in NSLBP patients. The current study’s results found significant improvement in pain, functional level, and spatio-temporal parameters of gait in both groups for all variables (p < 0.05). A significant difference has been detected between the control group and the study group in pain, disability, and CoG vertical displacement favouring the study group (p < 0.05). However, there were no statistically significant differences between groups for stride length, stride time, stride speed, and cadence in patients with NSLBP (p > 0.05).
The findings of the present study could be explained by many reasons. First, hip muscle weakness and hip muscle imbalance lead to lumbar pain in NSLBP. it has also been suggested that weak hip extensors and abductors contribute to LBP [27]. in addition, piriformis tightness from a lack of hip extensor strength contributes to LBP and limited hip rotatory motion [28]. As a result, it has been highlighted how essential it is to strengthen the hip extensors as well as abductors to reduce LBP [29]. Also, strengthening exercise can lead to improvement in functional scores which has an effect on pain as it disturbs a vicious cycle of pain sensitization and reinforcing disability [30]. Hip strengthening has been shown to improve the range of motion, decrease pain, enhance strength, and make movement easier for those with limited mobility [30]. Confidence improved and depression and fear related to LBP diminished when moving around without pain [31]. Therefore, patients can easily perform AdLs when they can walk around without experiencing pain [31]. improvements in spinal stability as well as decreased stress on the spine were seen in all participants when progressive hip strengthening exercises were added to lumbar stabilization exercises. This helped reduce the impairment encountered during everyday activities [32]. Muscle imbalance as well as abnormal movement patterns developed as a protective measure for injured areas, but they only increased pain in their daily activities [31, 32].
Many studies [28, 30, 33, 34] reported that adding hip strengthening exercises to the lumbar stabilization program improved disability and pain in patients with NSLBP. Similar to the findings of the current study, Lee et al. [33] found a significant decline in pain in patients having LBP following 6 weeks of lumbar stabilization exercises.
However, this study’s findings contradicted those of previous studies [35, 36]. Kendall et al. [35] conducted a study on NSLBP patients and found no significant improvement in pain and disability. The reason for this contradiction may be due to a reduced treatment session frequency as all participants received only one session per week. Fukuda et al. [36] concluded there were no significant differences in adding hip strength exercise to pain intensity and functional disability in NSLBP. The difference in the nature, frequency, and duration of treatment could account for this disparity. in the current study, hip strengthening exercises reduced the CoG significantly. The mechanism by which hip strengthening exercise induced that effect could be explained by the fact that the thoracolumbar fascia connects the hip muscles to the contralateral latissimus dorsi in the lower back, allowing the lumbar spine to act as a lever for transferring energy as well as loads to the lower limbs.37 Furthermore, the gluteal muscle group maintains pelvic stability, which in turn supports the spine. Therefore, individuals with LBP may benefit greatly from doing hip strengthening exercises in addition to their regular strength training for the trunk musculature [37, 38].
The gluteus medius makes up around 60% of the abductor’s cross-sectional area, making it the biggest of the abductor muscles. Because the gluteus medius plays an essential part throughout the single-leg stance time period, strengthening the hip abductors through targeted strength training is essential for performing functional lower limb motions. The lateral pelvis’ dynamic stability is largely controlled by the gluteus medius muscle [39], thus facilitating normal movement patterns, thereby improving balance by decreasing the vertical CoG displacement [29, 35]. When the center of gravity (CoG) is greatly altered, such as in a single-leg stance, the hip muscles, especially the gluteus maximus, are responsible for preserving pelvic stability as well as regulating the rotational motion of the lower extremities. Therefore, abnormal segmental motion of the lumbar spine during walking or standing may result from a lack of strength in these muscles, resulting in poor pelvic stability [38].
The findings of the present study were confirmed by many studies [29, 40, 41] demonstrating that the addition of hip strengthening exercises to the lumbar stabilization program improved normal movement patterns, dynamic stability, and decreased vertical CoG displacement in patients with NSLBP. Similarly, a study carried out by do and Yim [42] found that strengthening exercises for the hips not only improved single-stance performance, but also helped restore normal gait patterns and improved the strength of weak hip muscles, all of which had a positive effect on lumbar stability. on the contrary, the study findings come in contradiction with other studies [36, 43]. Fukuda et al. [36] did not support the positive effect of hip strengthening exercises on either clinical or kinematic outcomes in patients with NSLBP. This contradiction may be attributed to the difference in kinematic measuring tools and the nature, frequency, and duration of intervention. Bennell et al. [43] demonstrated a decline in pain and improvement in function after hip strengthening but detected no changes in mechanics during walking and joint loading while walking. This disparity is due to different methods of assessment and different populations.
Limitations
This study had limitations. First, the number of patients in this study was relatively limited which might pose a risk to positive findings. Second, the study participants were male subjects only. Additional studies are recommended to amend these limitations. Third, it would be helpful to follow the long-term impacts of this intervention, but we lack sufficient data to do so. Additional studies should aim to address this. Furthermore, the back muscle activities have not been investigated in the current study. Therefore, further studies should investigate muscle activity changes using electromyography.
Conclusions
Adding hip strengthening exercises to lumbar stabilization exercise programs can reduce pain, disability, and decrease CoG vertical displacement more than lumbar stabilization exercise programs alone in NSLBP patients. However, no differences were found in gait speed, cadence, stride length, or stride time.
Recommendations
From this trial, it is recommended that further studies compare males and females and undertake the use of larger sample sizes. For future research, it is proposed that a trial be performed to investigate the long-term effect of hip strengthening in NSLBP patients. Further studies should investigate muscle activity changes using electromyography.