Introduction
The annual global incidence of acute traumatic spinal cord lesions is 40 to 80 cases per million, including children [1, 2]. Robot-assisted devices can address some of the problems spinal cord injury patients encounter, including the Lokomat®Pro, a robot-assisted gait orthosis that makes it possible to walk on a treadmill without self-activity (see Figure 1). Settings stored in the device allow for many repetitions of exact movement sequences that are reproducible during each therapy session and crucial for the recovery process by stimulating neuroplasticity [3].
Compared to similar robot-assisted gait orthosis devices, the Lokomat®Pro has the most scientific articles published (309 as of 11. 05. 2016) [3]. The device increases motivation through immediate performance feedback on the patient screen and allows gait training using a high number of repetitions. Longer and more intensive training units lead to faster therapeutic success. The gait pattern, movement control and body relief can be individually adapted to the patient, and such training can also be carried out by people with severe motor problems. The general effects of exoskeletons include reduced muscle tone and activation and improved cardiovascular system performance. Furthermore, patients experience the feeling of flowing-walking again, and gravity positively impacts the skeleton, internal organs, optics and vestibular system. However, acyclic movements in everyday life, such as the transition upstairs or stumbling, cannot be practised [4]. Moreover, the device is very expensive, technically complicated, complex and heavy (ca. 1.000 kg) and requires a large space.
Studies on Lokomat®Pro involving adults with incomplete paraplegia are available, but studies on children with this clinical pathology have not yet been published [5–7]. Also, the influence of treadmill training on incontinence, which is present depending on the lesion level, has yet to be scientifically reviewed and published (as of November 2019).
Studies show that 75 % of patients with American Spinal injury Association Scale (ASiA-Scale) C or d severity can regain the ability to walk [8]. intensive and ongoing repetition, in the form of movement training, can increase the size of representative cortex areas [9, 10], with reorganisation of the neurospinal network possible [11]. indeed, synapses can change their transmission efficiency, remodel, or reform through different pathways, with each reshaping leading to neural network circuitry alterations [12].
Neuroplasticity describes organisational and structural brain changes in response to changing biological bases and demands. Through its various mechanisms, it is the principal prerequisite for functional recovery [13]. Even after complete transection of the spinal cord, Wolpaw and Lee [14] demonstrated task-specific spinal plasticity as early as 1989. Treatment with the Lokomat enables such movement therapy. in extended intervention units, it is feasible to consistently reproduce the same motion sequence by precisely storing the settings in the system, which would be difficult or impossible to do manually and require many therapists.
According to Karimi [15], upright standing and functional walking positively affect the human body and should be used for paraplegic patients. These effects include improving the cardiovascular system and bowel functions and preventing contractures. in addition, endurance sports influence the pelvic floor muscles [16], and treadmill therapy is indicated in patients with spasticity [1]. Thus, it can be assumed that such an approach can also reduce neurogenic bladder spasticity.
Increased quality of life and incontinence alleviation have been reported for other robotic-assisted gait systems, such as the ReWalk™ exoskeleton [17]. A research team led by Raab et al. [17] showed the influence of robot-assisted gait training (RAGT) on bladder control in an adult paraplegic patient. However, studies on the impact of RAGT on bladder control are rare in children with spinal cord injury (e.g., spina bifida).
Although it is well known that endurance sports such as Nordic walking, swimming, cycling and treadmill training have an influence on the pelvic floor muscles [16], Lokomat®Pro has not been explicitly used in either paediatric or adult settings to treat neurological disorders such as urinary incontinence. Nonetheless, Phelan et al. [18] showed that the Lokomat®Pro is suitable for treating children.
Since those with incomplete paraplegia cannot walk and suffer from incontinence, their quality of life is severely limited, and treatment could increase their health-related quality of life (HRQoL).
The aim of the study was to evaluate possible correlations between Lokomat®Pro treatment with independent locomotion in crawling, muscle function, and incontinence in a child with incomplete paraplegia at T10. one of the primary outcomes measured was whether Lokomat®Pro training had an impact on the pelvic floor and, thus, urinary control.
The clinical significance of the study for children with spinal cord injury lies in the improved bladder and bowel control and subsequent increase in HRQoL, which would provide therapists with another, albeit expensive, option to treat children with spinal cord injury.
Subject and methods
The controlled single case study was designed as an A-BA-B-A-E study. After written informed consent was confirmed, a seven-year-old boy with incomplete paraplegia at T10 due to meningoencephalitis (see Table 1) was observed over 17 weeks.
Table 1
Patient characteristics and ICD-10 codes (own recording)
The intervention took place at the Social Pediatric Center in Frankfurt (Germany) and included two 4-week intervention blocks (B) with the Lokomat, interrupted by a wash-out phase of 3 weeks (A), with a final 5-week wash-out phase (post measurements; prolonged from A to E). The wash-out phase was used to observe whether effects occurred during therapy that disappeared without therapy. There was no intervention during the wash-out phase because the child suffered from a broken leg. The same staff member of the Social Pediatric Centre and the research team always carried out the intervention and data collection, though none of the participants were blinded.
The child received standardised daily gait training of 60 min (including preparation and follow-up) with the Lokomat®Pro. The robot-assisted walking took place with massive weight reduction and was forward-facing. To increase motivation and compliance, the patient was able to play various gait training games on a video screen.
Follow-up included the Jandas five-step muscle function test, the 10-meter walk test (in crawling), surface sensitivity assessment, and the Patient Specific Functional Scale (PSFS) combined with the Canadian occupational Performance Measure (CoPMa-kids) at each measurement time (A). in addition, a bladder diary was kept by the mother throughout the observation period, and the Visual Analog Scale (VAS) determined pain before, during, and after each treatment by the physiotherapist to minimise the risk of injury.
Results
The Janda muscle function test showed a strong positive correlation between the therapy and torso muscle functions such as flexion, extension, rotation, hip flexion, bilateral abduction, and left adduction. indeed, there was an increase in the amount of movement in most directions during the intervention blocks. However, there was no impact on hip extension or adduction on the right side.
Crawling velocity doubled over the study period (0.041 m/s to 0.082 m/s) due to the Lokomat®Pro therapy. Unfortunately, the final measurement could not be performed because the subject sustained a tibia fracture during the school day in the last week of the second intervention block.
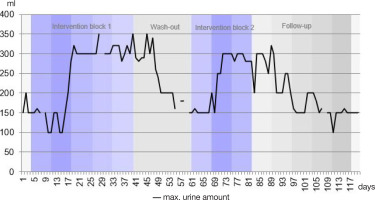
The filling volume of the neurogenic spastic bladder increased significantly with therapy and was maintained for about a week in the subsequent wash-out phases. Figure 2 shows the filling volume of the bladder across all days of the observation period.
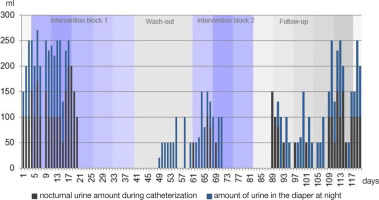
The number of catheterisation procedures required during the day decreased from a maximum of six to four, with nocturnal enuresis or catheterisation temporarily disappearing due to the therapy. However, urine amounts increased again after about a week without treatment (Figure 3). The stool consistency changed from a lumpy sausage shape to smooth and soft.
In intervention week two, there was a sharp increase in the maximum amount of urine. At first, the values fluctuated between 100 and 200 ml and reached 300 ml by the end of the second week. over the course of intervention block one, the values increased to a maximum of 350 ml and remained between 290 and 350 ml after completing the first block. Following a week’s break, values fell to a minimum of 150 ml. However, they increased again to a maximum of 300 ml after just 1 week of intervention in block two. Similar to the first intervention, the values remained largely constant for 1 week after the second block (except for day 83). Subsequently, the maximum bladder filling volume decreased again, though this occurred more slowly than after the first block. After 4 weeks without treatment, a mean value of 150 ml was again reached.
As in Figure 2, a similar course was seen for the amount of urine at night. initially, the patient woke up every night with a wet diaper (blue) and was catheterised (black). After the second week of intervention in block one, he was able to sleep through the night, and the bladder-filling volume was sufficient to prevent him from urinating at night. in the second week of the wash-out phase, nocturnal wetting reoccurred within the diaper, though the amount of urine was lower (between 20 and 150 ml) than at the beginning of intervention block one (between 100 and 250 ml). Furthermore, the urine volume was zero from about halfway through the second week of intervention block two, and it took until a week after completing the second block for urine to slowly increase again. However, initial values were reached after three and a half weeks without Lokomat®Pro treatment (wash-out phase).
In summary, when looking at the respective trends of muscle function in hip flexion and abduction on both sides and hip adduction on the left, a connection to Lokomat®Pro therapy was demonstrated. indeed, there was an increasing curve (right > left) with a subsequent falling graph for all movement directions during the intervention blocks.
The core muscles, related to flexion, extension, and rotation, improved over the entire observation period, which continued after the intervention, with trunk flexion increasing to the greatest extent.
There was a significant decrease in the time needed to crawl the required distance between the initial and intermediate measurements, with the time halving from 04:01 to 02:01 minutes. Meanwhile, crawling speed increased by 100 % from 0.041 m/s to 0.082 m/s. Although no Lokomat treatments took place during the wash-out phases, there was still further improvement in time and speed.
Our findings could be a sign of increased neuroplasticity, and since movement sequences seemed to improve and harmonise, they could be reproduced and carried out more quickly with repeated use. Using a regression graph of the power function and the logarithmic function, it can be shown that, with continued therapy, there will be a further increase in crawling speed and a reduction in the time required to cover ten metres. As such, it can be concluded that robot-assisted treadmill therapy had a positive effect on crawling speed in a child with incomplete spinal cord injury.
Discussion
Regarding the personal goals measured using the PSFS and the CoPMa-kids, the study participant experienced improved torso stability and could crawl, play ball, dress, and concentrate better (mothers’ answers in PSFS and CoPMa-kids).
For the VAS, the subject consistently scored a value of 0 (no pain). Nevertheless, it is essential to check the patient’s state of health at regular intervals, especially when sensitivity is so severely disturbed that injuries are not perceived. Borg-graefe et al. [19] indicated that skin redness, muscle pain, tendinopathies, and open skin lesions can occur due to the harness system. Therefore, the treating therapists must be made aware of possible risks.
Results of such examinations are of great importance, especially for affected persons, because current bladder treatment primarily involves spasm-reducing drugs that can be a long-term health burden. Effective conservative therapy could reduce this burden. The results of the current study confirm the findings of previous work demonstrating that RAGT can influence bladder control [17]. Since RAGT is used more and more frequently for children, its positive effects should also be investigated in larger case series or cohort studies [18, 19].
The feasibility of this robot-assisted therapy is not straightforward, because the acquisition costs for a Lokomat®Pro are very high (200,000 – 300,000 Euros) [20]. Furthermore, the device needs extensive space and cannot be easily moved once it is in place.
Whether the results of the current study apply to all children with an incomplete spinal cord injury remains unknown. As such, investigations must be carried out on more study participants of the same indication.
The significance of this study lies in the fact that non-surgical procedures to improve bladder control have been sought several times [21, 22], with the importance of incontinence in children greatly emphasised in many publications [23-26], including the immense suffering it causes among parents.
Technical options such as RAGT or treatments using virtual reality are very helpful for various clinical indications in adults and children [27, 28].
Limitations
The two major limitations of this study were the small number of subjects (n = 1) and the limited duration of 17 weeks. Another limitation was the tibia fracture suffered by the patient during the last intervention period.
Conclusions
Based on the available data, a hypothesis can be generated to state that the Lokomat®Pro is an effective treatment option for a seven-year-old child with incomplete paraplegia at T10 that increases independence by stabilising the trunk in the sitting position, strengthening the torso and hip muscles, reducing incontinence, and increasing locomotion speed during crawling.
Despite promising literature on Lokomat®Pro treatment for paraplegic adult patients, there is a clear lack of scientific evidence regarding its use in children with incomplete transection. Such literature should also be developed for children.
Since the present study was conducted with only one participant, only a first impression of the prospects of success can be demonstrated here. As such, further study should involve several participants with this clinical picture and investigate whether intensive therapy, such as twice-weekly treatment, would be sufficient to maintain its effects in the longer term.