Introduction
A hypertrophic scar (HS) is a skin condition defined by an abnormal increase in fibrous tissue containing disorganised collagen produced by skin fibroblasts. The HS experiences rapid growth 2 to 18 months after injury [1], with a prevalence of 8-67% for post-burn HS (PBhS). Both HSs and keloids can cause cosmetic and functional issues, including contractures, as well as subjective symptoms, such as itching and pain. These conditions significantly impact patients’ quality of life, physical well-being, and mental health [2, 3]. Although HS pathogenesis remains incompletely understood, the formation of HSs and keloids involves neural factors, cytokines, inflammation, and tension [4].
Treating HSs and keloids remains challenging since their cause is not fully understood. Available therapies include surgical and nonsurgical approaches, such as laser therapy, cryotherapy, compression, silicone sheet application, intrale- sional steroids, fluorouracil (5-FU), and botulinum toxin type A (BoNT-A) injections [5]. intralesional BoNT-A injections are considered a highly effective approach for preventing and treating HSs and keloids since they are delivered directly into the affected area [6]. BoNT-A is derived from anaerobic spore-forming bacteria and is used for medicinal and cosmetic applications [7]. Due to the limitations of BoNT-A injections, iontophoresis, a transdermal drug delivery system, is a beneficial option with several advantages. The method is painless and reduces damage by delivering drugs directly to the af fected area, and its effectiveness is increased by avoiding initial processing by the liver [8].
In addition to tap water iontophoresis, a long-standing treatment for hyperhidrosis, Clostridium BoNT-A was effectively delivered iontophoretically to patients with severe palmar hyperhidrosis [9]. BoNT-A affects HS by acting on wound tension, collagen, and fibroblasts. injecting BoNT-A directly into the lesion hinders fibroblast proliferation and decreases the production of connective tissue growth factor protein [10].
Accordingly, this study aimed to assess the effectiveness of BoNT-A iontophoresis in improving PBHS physical characteristics and minimising HS thickness.
Subjects and methods
Study design
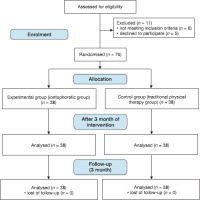
This single-blinded randomised controlled trial was performed between August 2022 and December 2023, with the investigators blinded. The 76 participants were randomly divided into an experimental group (iontophoretic group, n = 38) and a control group (traditional group, n = 38) and followed a three-month program with two sessions/week. The iontophoretic group received BoNT-A iontophoresis once a month, plus traditional physical therapy (TPT, stretching exercises and scar massage), while the traditional group received a TPT programme only.
Randomisation
An unbiased patient selected the groups by blind-drawing numbers from sealed envelopes produced through a random number generator. Randomisation was limited to permuted blocks to guarantee that the numbers allotted to both groups were equal. Sequences allotted to patients were placed in envelopes containing the group allocations.
Patients
The study included 76 patients with PBHSs aged 20-40 years [with mean ± standard deviation (SD) of 31.74 ± 6.31 in the iontophoretic group and 31.21 ± 7.11 in the traditional group]. The inclusion criteria were (1) both sexes aged 20-40 years, (2) HS 3-6 months after burn healing and scar surface area < 40 cm2, (3) total body surface area damage < 20%, and (4) thermal burns. All patients signed informed consent before participation. Participants were excluded from iontophoresis for (a) history of cardiac arrhythmias, (b) presence of cardiac pacemakers, (c) orthopaedic implants, (d) skin lesions or impaired sensation, (e) pregnancy or breastfeeding, and (f) diabetes mellitus. Exclusion criteria for drug administration included [12] (a) known hypersensitivity or negative reactions to BoNT-A, (b) BoNT-A treatment within six months of the study starting, and (c) psychiatric or neurological conditions like myasthenia gravis.
Sample size determination
The sample size was calculated using G*POWER version 3.1.9.2 (Franz Faul, Universitat Kiel, Germany), employing a = 0.05, power = 90%, effect size = 0.76, and an allocation ratio of N2/N1 = 1. The calculation was based on Vancouver Scar Scale (VSS) data from a previous study by Elshahed et al. [12], who reported a significant effect of BoNT-A compared with the control in treating HSs. Herein, the required sample size was 38 subjects/group.
Assessment
At baseline, all patients underwent a comprehensive evaluation that included demographic data, general medical history, and extensive information on their scars, such as the aetiology, duration, location, and associated symptoms.
Scar evaluation was conducted using the Patient Scar Assessment Scale (PSAS) and Observer Scar Assessment Scale (OSAS). Furthermore, all lesions were evaluated using high-resolution ultrasound to quantify scar thickness.
Patient and Observer Scar Assessment Scale
The Patient and Observer Scar Assessment Scale (POSAS) was created to assess various scars, with POSAS version 2 comprising the OSAS and PSAS. The patient scale evaluates scar features such as colour, pliability, thickness, relief, itching, and discomfort, while the observer scale evaluates vascularity, pigmentation, thickness, relief, pliability, and surface area. items are rated on a scale from 1 (normal skin) to 10 (worst scar imaginable), with the overall score on the observer scale calculated from the sum of the six components. Category boxes are included for every item [13, 14].
This trial used the Arabic version of POSAS, a straightforward, reliable, and valid tool for evaluating burn scars in the Egyptian population [15]. Participants answered the questionnaire pre-treatment, after three months (post-i), and after six months (post-ii).
Ultrasonography
Ultrasonography evaluated tissue elasticity, stiffness, and structure at the same time using elastography and B-mode. The HS thickness was measured using B mode [16], while a GE Voluson S8 high-resolution ultrasonography machine measured scar thickness, with measurements taken from the thickest spot if the scar was uneven. All evaluations were conducted with a 12-MHz linear probe. Scar thickness was measured pre-treatment, after three months, and after six months.
Treatment
Both groups followed a three-month treatment program. in the iontophoretic group, the scar area was prepared using saline, and scar tracing was used to calculate the surface area to indicate the required dose of BoNT-A (Allergan®, CA, USA). The Botox product consisted of a 100 U vacuum-dried powder contained in a single-use vial and reconstituted through dilution in 2 mL of preservative-free sterile saline (0.9%). The product was administered to a cathode using a syringe at a concentration of 2.5 iU/cm2 (equivalent to 5 U/0.1 ml). An active electrode was positioned just above the scarred area, and a dispersive electrode was positioned on the skin six inches from the active electrode. The dose was set at 40-80 mA/ min on a PM850 Phoresor® ii Auto device (iOMED, Barcelona, Spain). The current intensity increased progressively from 0 to 4 mA, depending on the subject’s tolerance. The device computed the necessary time for the chosen dose automatically. The iontophoretic device used a direct current in its application. Treatment was undertaken once per month for three months.
Both groups underwent a TPT program (stretching exercises and scar massage) two times per week for three months. Stretching exercises are important for improving post-burn contractures because manual stretching techniques influence joint function retention and general range of motion (ROM). Furthermore, soft tissue mobilisation may reduce scar thickness and improve the pliability of burn contractures. Manual passive stretching was applied over the scar area, especially when the scar crossed a joint. The stretching hold period was 30-60 s and included three sets of 10 repetitions [17].
Scar massage improves skin qualities such as flexibility, adhesions, pruritus, and pain, increasing skin mobility. Massage can commence as soon as the scar tissue is epitheli- alised and solid, which allows for the support of specific manual techniques. Techniques applied to the scar included (a) Morice orthodermic stretching, which involves applying consistent stretching pressure that is supported in the opposite retraction direction. The second technique, punctual crushing, uses vertical controlled pressure applied by the pulp of one or more fingers that can circulate without friction or lifting of fingers. The third technique used static folds created by pinching between two fingers or by pressing together with both hands, with no frictional movements. The final technique, palpate-rolling, uses static folds that transform into a rolled fold to greatly reduce the severity of deep plans and fibrosis scars [18]. The traditional group only received the TPT for the same duration as the BoNT-A treatment.
Statistical analysis
Statistical analysis employed SPSS version 25 for Windows (iBM Corp., NY, USA). An unpaired t-test compared the ages between groups, while a chi-squared test (Fisher’s exact test) compared sex, scar site, and skin type distribution among different groups. The Shapiro-Wilk test assessed data distribution, while Levene's test evaluated homogeneity of variance. A mixed-model multivariate analysis of variance (MANOVA) examined the impact of therapy on scar thickness and PO- SAS. Post hoc testing with Bonferroni's correction was conducted for multiple comparisons. Statistical significance was indicated by p < 0.05. The intention was to treat the analysis with multiple imputation methods to account for the missing data. There was no dropout or missing data in this study.
Results
Subject characteristics
The results revealed a non-significant difference in age, scar duration, sex, skin type, and scar site distribution between groups (p > 0.05, Table 1).
Table 1
Subject characteristic
According to the Fitzpatrick Skin Phototype Classification, Egyptian people have skin types ii and iV. Skin type iii is medium white skin, which sometimes burns tans slowly, and skin type iV is moderate brown skin that burns minimally and tans readily [19].
Treatment effect on scar thickness and Patient and observer Scar Assessment Scale
Mixed-model MANoVA results indicated a significant treatment and time interaction effect (F = 121.54, p = 0.001). The findings showed that treatment (F = 4.24, p = 0.002) and time (F = 314.48, p = 0.001) had a significant main effect.
Within-group comparisons
The results showed a significant reduction in scar thickness, oSAS total score and general opinion, and PSAS total score and general opinion in both groups at post-i and ii compared to pre-treatment (p < 0.001). These parameters were also significantly lower in the iontophoretic group at post-ii than at post-i (p < 0.001), with non-significant differences between post-i and ii in the control (p > 0.05; Tables 2 and 3).
Table 2
Scar thickness pre-treatment, post-i, and post-ii
Table 3
Patient and observer Scar Assessment Scale pre-treatment, post-i, and post-ii
Between-group comparison
Scar thickness and PoSAS exhibited non-significant differences between both groups pre-treatment (p > 0.05). Moreover, scar thickness, oSAS total score and general opinion, and PSAS total score and general opinion of the iontopho- retic group exhibited a significant reduction compared to the control at post-i and ii (p < 0.05; Tables 2 and 3).
Discussion
Previous research has investigated intralesional BoNT-A injection for different HSs and keloids. Accordingly, this study aimed to determine the effect of BoNT-A iontophoresis in PBHS treatment, which was documented through ultrasonography and PoSAS assessments. This study is the first to evaluate the effects of BoNT-A iontophoresis in treating PBHSs.
The iontophoretic group achieved significant enhancement in scar thickness post-i and ii in comparison to pretreatment, post-ii, and post-i, with percentage increases of 13.64, 24.43, and 12.50%, as well as 5.29, 5.85, and 0.59% for the traditional group, respectively.
Osas total score improved by 17.92, 30.14, and 14.89% in the iontophoretic group and by 7.13, 7.95, and 0.88% in the traditional group. oSAS general opinion was 25.16, 40.30, and 20.22% in the iontophoretic group and 17.17, 20.17, and 3.62% in the traditional group.
Psas total score improved by 15.47, 28.86, and 15.84% in the iontophoretic group and by 6.16, 6.58, and 0.45% in the traditional group. Finally, PSAS general opinion improved in the iontophoretic group by 19.48, 36.50, and 21.14%, and 10.14, 13.72, and 3.70% in the traditional group.
These results are in line with several experiments, such as Tawfik and Ali [10], who randomised 15 children with PBHS and keloid scars to receive an intralesional BoNT injection (Neuronox, 100 U; Medytox, Kak-rl, ochang-myeon, Cheong- won-gun, Chungcheongbuk-do, Korea) on one area of the HS/keloid, while using another area as a control. They used VSS alongside a skin analysis camera system, revealing dramatically improved scar lesion vascularity, pliability, and height [10].
Khatery et al. [20] evaluated the clinical and histopatho- logical impacts of monthly BoNT-A injections on keloids and HSs over three months. They found marked improvements in VSS, oSAS, PSAS, and histopathologic findings after each injection session, as well as at the three and six-month followup, compared to baseline (p < 0.001 for each) [20].
Elshahed et al. [12] evaluated the safety and efficacy of BoNT-A injection in HSs through a split scar. Thirty 1-15-year- old patients with old scars were treated with BoNT-A or 0.9% normal saline once a month for three months. The mean VSS score decreased in the BoNT-A-treated half of the scars posttreatment, with non-significant changes in the control half. The BoNT-A-treated group demonstrated substantial clinical and cosmetic improvement [12].
Hu et al. [21] administered BoNT-A to one side of surgical wound closures directly post-surgery. The results indicated a significant difference in the VSS height score in the injected group based on the visual analogue scale and scar width measurement results. Furthermore, early post-surgical BoNT-A injections enhanced the appearance and reduced the width of facial surgery scars on the treated sides [21].
Shaarawy et al. [22] examined the effectiveness and safety of injecting BoNT-A and corticosteroids directly into keloids. The study demonstrated reductions in lesion volume posttherapy, with 82.7% and 79.2%, respectively. Moreover, the steroid group achieved improvements in lesion softening, with the lesions exhibiting reductions in height and redness score, though there was no distinction between both groups. Subjective complaints decreased, especially in the BoNT-A group. Meanwhile, three patients exhibited skin atrophy and telangiectasia in the steroid group. As such, intralesional BoNT-A demonstrated efficacy and safety comparable to in- tralesional corticosteroids, showing considerable improvement in objective measures and keloid volume [22].
The previous investigations have validated our statistical findings and affirmed BoNT-A’s effectiveness in scar treatment, which could be attributed to the impact of BoNT-A on wound healing. The crucial aspect that influences the ultimate cosmetic appearance of a scar is the strain exerted on the wound’s margins throughout healing, which might result in unfavourable scars through direct (mechanical) and indirect (chemical) means. Perpendicular tension on wound margins can mechanically strain wound muscles, disrupting the normal healing process and causing HSs and keloids to form. Local injection of BoNT-A induces transient paralysis in the affected muscles, leading to immobilisation and decreased perpendicular tension. indeed, BoNT-A enables almost total removal of dynamic muscle tension on the wound while it heals [6].
One further hypothesis on how BoNT-A affects HSs and keloids is that it influences the cell cycle distribution of fibroblasts. The excessive growth of fibroblasts contributes to the development of HSs and keloids. BoNT-A can influence fibroblast activity by changing apoptotic, migratory, and fibrotic pathways in pathological scars. This can lead to a slower proliferation rate, reduced secretion of biologically active substances, and decreased extracellular matrix and collagen synthesis, ultimately improving HSs and their appearance [23]. Moreover, BoNT-A can cause paralysis of the wound edges, reducing tension vectors until collagen matures [6].
An alternative interpretation of our results is that iontophoresis may address the barrier resistance of the scarred skin epidermis and specifically target the scar area [24]. iontophoresis is an established method for improving transcutaneous drug delivery, offering the benefits of being noninva- sive, painless, and externally regulated. Nanocarriers and electrical currents utilise hair follicles as a preferred entry mechanism, making their combination beneficial for improving drug delivery to the skin [25]. iontophoresis has several advantages over other transdermal techniques, such as its ability to transport small and large molecules, ease of use, possible self-administration, and no cell damage. Still, iontophoresis may cause skin irritation, and incorrect placement of electrodes may lead to the risk of burn [26]. Several drugs, such as potassium iodide, hydrocortisone, and acetic acid, were administrated iontophoretically for scar treatment, significantly improving different types of scars [27-29].
Bont-A iontophoresis helped treat PBHSs and demonstrated superiority over TPT programs for HS improvement. Our findings are consistent with current research and highlight the relevance of using BoNT-A iontophoresis clinically for PBHS improvement.
Limitations
The study’s limited sample size of 76 patients and the narrow age range of 20-40 years may restrict the applicability of the results to larger populations with PBHSs. The follow-up period, lasting three months, may not be sufficient to assess the long-term effectiveness and safety of BoNT-A iontophoresis. Furthermore, additional objective measurements and detailed adverse event monitoring alongside scar thickness and POSAS will enhance therapy efficacy and safety evaluation. Moreover, further studies are required to investigate BoNT iontophoresis in different types of scars. However, the high cost of BoNT-A may be a limitation, especially in a large sample.
Conclusions
Intralesional injection of drugs has several drawbacks. iontophoresis is an alternative transdermal delivery method for several drugs, with the advantage of being noninvasive, painless, and externally controlled. Furthermore, it allows for the delivery of polar molecules and high molecular weight compounds such as peptides and proteins. Based on the study findings, BoNT-A can be introduced iontophoretically to improve PBHS characteristics and reduce its thickness, with effects lasting for around three months.