Introduction
Breast cancer is the most commonly diagnosed cancer and the primary cause of death among cancer-affected women worldwide, with more than 1.8 million patients diagnosed globally every year. Recent years have seen a considerable increase in breast cancer survival rates due to early screening facilities, improved medical treatment options, and disease identification. Over 80% of those affected can be cured and usually undergo mastectomy [either modified radical mastectomy (MRM) or breast-conserving therapy (BCT)] [1,2].
With such increased survival rates, one of the associated side effects of surgery is the development of secondary lymphedema (SL). The excision of lymph nodes surgically, or along with radiation therapy, is likely to result in SL. The term “arm swelling and dysfunction” is commonly used to charac terise SL, defined as an increase in arm circumference of more than 2 cm or an accumulation of protein-rich fluid in areas with in the first six to 12 months. Moreover, the risk rate increased between 36 and 48 months for those who underwent a sentinel lymph node biopsy and regional lymph node irradiation [3].
Shoulder arm exercises are necessary following surgical treatment to prevent lymphedema and improve shoulder joint movement and strength, which enhance shoulder function and promote quality of life (QoL). Evidence suggests that patients should initially exercise slowly and then introduce light weights, with a few repetitions, increasing gradually and stopping if painful [1]. Resisted muscle training is critical to attenuating and correcting muscular dysfunction during cancer therapy because preventing muscle dysfunction is a priority [4]. Such exercises include scapula-oriented exercises for eight weeks, which involve stretching and strengthening for 40 min, with a five-minute warm-up and cool-down. This approach may reduce pain, improve muscle strength, and enhance QoL in breast cancer survivors [5, 6]. To the best of our knowledge, there is a lack of robust randomised clinical trials in india on the effects of scapular and shoulder exercises in SL prevention among those undergoing breast cancer surgery. Hence, this study was designed to analyse the impact of scapular and shoulder muscle strengthening exercises in improving shoulder function and preventing upper limb SL development.
The primary objective of this study is to determine the effect of scapular and shoulder exercises on muscle strength, range of motion, arm circumference, arm volume, pain, and shoulder disability among women who underwent breast cancer surgery. The secondary objective is to analyse the effect of scapular and strengthening exercises on improving QoL among women who underwent breast cancer surgery and find the relationship between shoulder disability and QoL. Hence, we hypothesised that scapular and shoulder strengthening exercises will improve shoulder function, prevent SL development, and enhance QoL after breast cancer surgery.
Subjects and methods
This study protocol explains the method for a randomised clinical trial as per SPiRiT (the Standard Protocol items: Recommendations for interventional Trials) [7].
Trial design and study setting
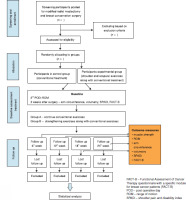
The protocol adopted a single-blinded randomised clinical trial with two parallel groups design and has a 1:1 allocation ratio for receiving the treatment programme. Screening of participants, allocation, assessments, and interventions will be conducted at the departments of oncology and surgery at the PSG Hospitals, Tamil Nadu, india.
Trial recruitment and allocation
Participants will be recruited from the oncology and general surgery departments of the PSG Hospitals. Participants who undergo MRM will be referred to a physiotherapist (DV) from both departments, and the therapist will teach the conventional exercise protocol. The primary investigator (MV) will teach the strengthening exercises to the interventional group. in order to achieve the expected sample size and adequate participant enrolment, a standardised screening of participants will be carried out based on the inclusion and exclusion criteria.
Study participants
Fifty-eight female participants aged between 25 and 65 years, who will undergo either MRM or BS unilaterally with axillary lymph node dissection for the first time, will be included in this study. Participants who undergo neoadjuvant chemotherapy or adjunct chemotherapy and radiation and those who come under a comprehensive health insurance scheme (sponsored by the state government) will also be included. Participants should agree to be monitored via video call during follow-up assessment and be willing to participate in the study. Criteria for exclusion are cardiovascular symptoms at low-intensity activities [< 3 metabolic equivalents (METs)], impaired cognition, or musculoskeletal, rheumatic or any other conditions precluding their participation.
Sample size
The comparison of the mean formula calculated the sample size using the mean and standard deviation (SD) values of shoulder external rotator muscle strength from a previous study [5] (sample size = 2SD2 x (Z1-/2+ZP) /d2, where Z1—1/2 is the standard normal variate [1.96 at 5% error], Zp is 0.842 at 80% power, SD = the SD from previous studies, and d = the difference between the mean values of the interventional group. Hence, substituting these values [SD (a) of 13.2; pre-test mean value = 54.5 joules; post-test mean value = 65.1 joules; mean difference = 10.6 joules], sample size = 2 (13.2)2 x (1.96 + 0.84)2/ (10.6)2 = 24, an approximate sample size of 24 participants in each group was calculated. Considering a 20% dropout (10 participants), the sample size in each group will be 29 per group. Therefore, this study needs a total sample size of 58 participants.
Procedure
Participants referred by a surgical oncologist and a general surgeon will be screened by the primary investigator (MV) for their eligibility to participate in the trial. A patient information pamphlet about the study, postoperative exercises, skin care, and safety during the trial will be provided to the participants, and informed consent will be obtained.
Randomisation
Eligible participants will be allocated randomly to an interventional group or a control group with the help of a web- based tool (www.sealedenvelope.com). The Block randomisation method will be adopted, with ten blocks of six participants each and a 1:1 allocation ratio. The results will be stored in sealed envelopes and maintained confidentially by the investigator (VR) conducting the randomisation procedure. Then, the participants will be allotted to groups according to the sequence generated. The researcher who generates the randomisation sequence will not be involved in the recruitment, assessment, or intervention. The outcome assessor (DR) will be blinded to the allocated groups. Since the therapists and participants will be directly involved in the study, they cannot be blinded to the intervention after the group assignment.
Demographic details and baseline outcome assessments
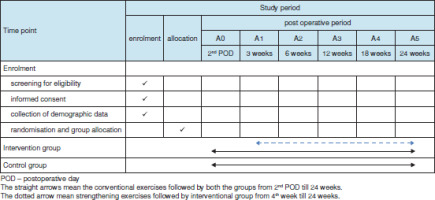
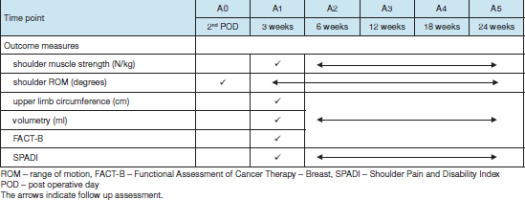
Demographic details of all participants will be recorded and tabulated, as shown in Table 1. Primary outcome variables include shoulder and scapular muscle strength, shoulder range of motion, upper limb circumference, volumetry, shoulder pain, and disability. The secondary outcome variable is QoL assessment. A baseline shoulder range of motion assessment will be taken on the second postoperative day (A0), while a baseline assessment of all other outcome measures will be taken at the end of the third week after surgery (A1).
Table 1
Details of demographic characteristics
The participants will be followed up four times after surgery, including weeks six (A2), 12 (A3), 18 (A4) and 24 (A5) (Figure 1). The protocols for patient assessment, group allocation, and treatment programme are outlined in Tables 2 and 3.
Intervention
A Template for Intervention Description and Replication (TIDieR) checklist [8] was implemented to describe the study intervention (Table 4).
Participant withdrawal
The participants will discontinue the study if any adverse events occur and can also leave without consequences if they withdraw their consent. A participant can be removed from the study by the primary investigator due to unavoidable circumstances. The reasons for withdrawal will be documented.
Adverse events during the trial
Adverse events related to exercise might cause fatigue or pain. Participants will be given a washout period of two to four days following chemotherapy for their fatigue and pain to settle down, after which they will resume their exercise programme. If a participant scores over five on the visual analogue scale (VAS) during admission, a transcutaneous electrical nerve stimulator (TENS) will be used for pain relief. Adverse events present during assessment and follow-up sessions will be documented.
Outcome measures
Primary outcome measures
Shoulder and scapular muscle strength
The strength assessment for shoulder flexors, abductors, internal and external rotators, serratus anterior, upper trapezius, and latissimus dorsi will be measured (in kilograms and represented in Newtons) using a baseline push-pull dynamometer (model 12-0300; Fabrication Enterprises, NY, USA). We conducted a pilot study to assess the inter-rater reliability of a baseline push-pull dynamometer on healthy individuals. We found that measuring scapular and shoulder muscle strength had moderate to excellent reliability, with an intraclass correlation coefficient [ICC] of 0.78 (95% confidence interval [CI] = 0.57, 0.89) for shoulder flexor strength and 0.79 (95% CI = 0.59, 0.89) for shoulder abductor strength.
Shoulder range of motion
The shoulder range of motion will be measured in degrees using a universal goniometer (Mediguard, Uttar Pradesh, india). The test-retest reliability was indicated by an iCC between 0.95 and 0.98 (p < 0.01) [12]. The convenience of using the universal goniometer and its availability make it easier to use [12].
Upper limb circumference measurement
The arm volume, measured by upper limb circumference, will be marked in 4 cm segments from the ulnar styloid process towards the axilla (» 0 to 40 cm) and 4 and 8 cm down the hand from the ulnar styloid process. The definition of lymphedema is an increase of more than 10% in volume, i.e., > 2 cm in the circumference between the affected and normal upper limbs [13]. Pooled iCC data from studies measuring lymphedema using inch tape measurement of the upper extremities showed an intra-rater reliability value of 0.99 (95% Ci = 0.99, 0.99) and an inter-rater reliability value of 0.98 (95% Ci = 0.98, 0.98) [14].
Volumetry
The volume of the upper limb will be measured by immersing the affected and unaffected upper limbs in a container of water, with the displacement volume measured at around 5 ml. A customised volumetric assessment water can was devised and modified so that the can is fixed with a tap on top. A mark will be made 40 cm from the ulnar styloid process on both arms, and the participant will immerse the upper limb in the water-filled container until the water level reaches the mark made on the arm. The tap will be kept open when the participant starts immersing their upper limb to collect the displaced water in a beaker and will be closed when the displacement of water stops. The water collected in the beaker will then be measured, and the values for each upper limb compared. Lymphedema is defined as an increase of more than 10% in volume, i.e., more than 200 ml in arm volume between the affected and normal upper limbs [13]. Pooled iCC data of studies measuring lymphedema of the upper extremities using the voltmeter had an intra-rater and inter-rater reliability of 0.99 (95% Ci = 0.99, 0.99) [14].
Shoulder pain and disability index
The Shoulder Pain and disability index (SPADI is a questionnaire relating to pain and the difficulty that a participant has with day-to-day activities involving shoulder movements for upper extremity use. The means of the pain and disability subscales are averaged to arrive at a total score ranging between 0 (the best) and 100 (the worst). The Tamil version of SPADi, developed by Jeldi et al. [15], will be used for our study. The scale has good internal consistency for the pain (a = 0.859) and disability (a = 0.895) subscales. Moreover, the test-retest reliability has been reported to be excellent for pain (iCC= 0.989; minimal detectable change - 6.27) and disability (iCC = 0.990; minimal detectable change - 6.25) [16]. Secondary outcome measure
Functional Assessment of Cancer Therapy – Breast Version 4 questionnaire
Participant QoL will be evaluated using the Functional Assessment of Cancer Therapy - Breast (FACT-B) questionnaire (version 4, in Tamil) [17]. The validated questionnaire has physical, social, emotional, and functional well-being subscales and also includes questions related to several more concerns about breast cancer. FACT-B contains 37 questions and is divided into FACT-general (FACT-G) and a disease- specific domain for breast cancer (BCS).
Fact-B-Total is the total score of all subscales, ranging from 0 to 148, with higher scores indicating greater QoL [16]. The FACT B+4 subscale has shown good internal consistency (Cronbach’s alpha = 0.62 to 0.88) and high test-retest reliability (0.97) [17].
Outcome assessments
There will be five evaluation time periods in this study, including baseline at the third week (except for shoulder ROM measured on the second postoperative day) and post-intervention at weeks 6, 12, 18, and 24 after surgery. An assessor blinded to the intervention and trained to perform the assessments will document the outcome measures, though the SPADi and FACT-B questionnaires will be answered by the participants. The timeline for outcome measure assessment is explained in Table 2.
Data analysis
The collected data will be statistically analysed using Statistical Package for the Social Sciences (SPSS) version 16.0 (iBM Corp., NY, USA). Participant demographic and clinical characteristics will be analysed through descriptive statistics, while the outcome variables in the two treatment groups will be described with mean ± SD. The Shapiro-Wilk test and histograms will evaluate data normality, and an independent t-test will assess if the baseline data are similar between the two groups (p > 0.05). Between-group differences for the outcome measures of interest, including all time points, will be assessed using a mixed-model analysis of variance (ANOVA). The incidence of SL will be reported as a percentage, and between-group differences will be assessed using the chi- squared test. Pearson’s correlation coefficient will calculate the relationship between QoL and shoulder disability for each group. For missing data, an intention-to-treat analysis will be used and handled through multiple imputations. The level of significance will be set at < 0.05.
Discussion
Impaired muscle function can result from breast cancer surgery and its treatment through atrophy and loss of strength. Muscle weakness reduces a patient’s ability to do routine day- to-day activities and stay independent during and following treatment [18]. The present study will analyse the effects of scapular and shoulder muscle exercises in improving shoulder range of motion, preventing SL, and improving QOL after breast cancer surgeries among women in an indian setting. So far, only shoulder range of motion exercises have been emphasised following surgery. implementing strengthening exercises will improve shoulder range of motion and prevent muscle atrophy and SL development, thereby contributing to a good functional recovery. Carolyn et al. [11] demonstrated that one to three pounds of weight should be used initially for strength training in mastectomy patients, though it may cause mild fatigue at the end of 10 repetitions. in our study, we will commence strength training with 0.5 kg, which is approximately one pound. Progression should be made by increasing the weight, number of sets, or weekly frequency [11]. in this study, we will progress by starting the exercise with a 500 ml water-filled bottle, slowly increasing to 750 ml and 1 l bottles. To achieve a considerable improvement in the study outcomes, treatment duration will be 24 weeks (four sessions per day; 20 min per session). The results of the several outcome measures of interest will be compared between the two groups to evaluate the effects of scapular and shoulder muscle strengthening exercises in the prevention of SL, among others. The findings of the study will be helpful in setting up a better exercise protocol to prevent SL and improve QoL among indian women following breast cancer surgery.
Limitations
The partially supervised exercise programme is one of the limitations of the trial, as the participants will only be monitored through video calls. Though a convenient time for monitoring may pose a challenge to the therapist, measures will be taken to motivate the participants by explaining the uses of the study intervention to them and their caretakers. Those who lack adherence to a regular exercise programme will be encouraged by being shown clinical pictures and educational videos related to the development of lymphedema and its consequences. Participants will be given feedback on their muscle strength, shoulder range of motion, arm circumference, arm volumetric assessment, shoulder pain and disability, and QoL scores at each assessment to encourage their performance and adherence to the exercise programme. Future studies with an extended follow-up period are warranted to investigate the long-term effects of the intervention and the occurrence of lymphedema.
Data collection and management
The data of all participants will be maintained by the primary investigator (MV), though all investigators will be able to access the data. Details of the participant’s name, address, history, and results of outcome measures will be kept confidential.
Dissemination of results
The results of the study will be published in journals, at conferences, and on the Clinical Trial Registry of india. The participants will also be informed of the study outcomes during their follow-up. Researchers who contribute to trial design and conduct, data interpretation, and reporting will be considered for authorship.