Introduction
De Quervain tenosynovitis (QT), first described by Swiss surgeon Fritz de Quervain, is an inflammatory condition characterised by tendon entrapment within the first extensor compartment of the wrist (dorsal first compartment) [1,2]. QT is characterised by thickening and degeneration of the tendon sheaths surrounding the abductor pollicis longus and extensor pollicis brevis as they traverse the osteofibrous tunnel along the radial styloid in the distal wrist [1,3].
Risk factors for QT include female gender, age over 40, manual labour, and black race [4]. QT prevalence is 0.5 % in males and 1.3 % in females, peaking between the ages of forty and fifty [4, 5]. Additionally, soft tissue oedema, fluid retention, and ligamentous laxity, commonly affecting women during the last trimester of pregnancy or the postpartum period, contribute to this condition [1,4].
Medical history and physical examination findings combine to diagnose QT. Patients typically present with pain and oedema localised to the radial styloid region, which is exacerbated by manoeuvres involving ulnar deviation and flexion of the thumb metacarpophalangeal joint [1,6]. The physical exam commonly includes the Finkelstein test, which reproduces pain when performing these movements and can reveal swelling and tenderness at the radial styloid process [2, 4]. Ultrasonography (USG) aids in diagnosis, particularly in cases requiring corticosteroid injections, by enhancing in- jectlon success rates and assisting In preoperative planning for surgical interventions [1,7].
Qt management varies depending on its severity, with conservative options such as nonsteroidal anti-inflammatory drugs and thumb spica splinting providing effective pain management by preventing thumb metacarpophalangeal flexion and ulnar deviation of the wrist [7, 8]. Corticosteroid infiltration into the first extensor compartment prolongs relief in 80% of cases [7], while surgical release of the first compartment, followed by a period of splint immobilisation, is recommended when conservative treatment fails [1,8].
Physical therapy, a conservative QT treatment option, aims to alleviate pain and improve functionality by encompassing various approaches such as conventional ultrasound (US), manual therapy, therapeutic exercises, and low-level laser therapy (LLLT) [7-9]. LLLT is a non-invasive and painless therapeutic resource that has demonstrated efficacy in managing acute and chronic pain associated with musculoskeletal disorders. Additionally, it has shown effectiveness in controlling inflammation and facilitating soft tissue healing [10-12].
Lllt operates on the principle of light amplification by stimulated emission of radiation (LASER), generating monochromatic light, typically within the red or near-infrared spectrum. LLLT enables the stimulation or inhibition of biological processes through photobiomodulation, which varies depending on the energy delivered to the tissues. The therapy uses class lllb lasers with power below 0.5 W [10, 11] to avoid the heating of biological tissues, unlike high-power lasers (class IV) [13]. LLLT reduces inflammation and promotes collagen synthesis and angiogenesis [11]. Additionally, activation of enzymes in the respiratory chain (complex IV) stimulates adenosine trisphosphate (ATP), deoxyribonucleic acid (DNA), and ribonucleic acid (RNA) synthesis and cellular metabolism [11, 12, 14]. The physiological mechanisms underlying LLLT-induced analgesia include decreased nociceptive nerve conduction, p-endorphin release, and inflammatory mediator reduction [11-13].
Considering these effects, studies on many musculoskeletal disorders [10, 11, 15], including tendinopathies like QT, have proposed LLLT as a beneficial treatment for reducing pain and inflammation and improving functionality [16-18]. Furthermore, many patients prefer conservative treatment options over surgical release, mainly due to the associated recovery times. However, despite the analgesic benefits of LLLT, the number of clinical trials supporting its effectiveness compared to other treatments seems to be limited. Hence, this systematic review (SR) aimed to gather and assess the existing scientific evidence on the analgesic effects of LLLT in patients diagnosed with QT.
Subjects and methods
Type of study and design
This quantitative SR employed an observational, retrospective, and secondary design. The review adhered to the guidelines outlined by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA 2020) [19]. Additionally, the SR was registered at the International Prospective Register of Systematic Reviews (PROSPERO) by the National Institute for Health Research with the registration number CRD42024530324 (registration date of March 28th 2024) [20].
Selection criteria
The study adhered to the PICOS approach (patient, intervention, comparison, outcome, and type of studies), focusing on QT patients who received LLLT alone or in addition to other physical therapy treatments that were compared with other physical therapy interventions, medical treatments, or placebos. The primary outcome measure was pain intensity assessment using scales such as the visual analogue scale (VAS) or the numeric pain rating scale (NPRS). Secondary outcomes encompassed alterations in grip strength and disability, evaluated through scales or questionnaires such as the Disabilities of the Arm, Shoulder, and Hand (DASH) questionnaire. The studies analysed included randomised and nonrandomised clinical trials in English or Spanish.
The exclusion criteria comprised case studies, literature reviews, SRs on LLLT unrelated to this study, research involving individuals with QT coexisting with other musculoskeletal disorders or neurological conditions, and studies characterised by incomplete or inaccessible data.
To ascertain eligibility, two researchers (TCH and MJG)) independently evaluated potential studies by scrutinising titles, abstracts, and full texts. A third researcher (HDB) resolved any selection disparities.
Literature review
Pubmed, Scopus, Web of Science (WoS), EBSCOhost, Embase, Cochrane, and the Physiotherapy Evidence Database (PEDro) databases. Additionally, the grey literature was explored through the Google Scholar search engine [21].
Keywords derived from the Medical Subject Headings (MeSH) dictionary were used, including “Lasers,” “Laser Therapy, ” “Phototherapy, ” “Low-Level Light Therapy, ” “Laser Class IIIb, ” “Musculoskeletal Pain, ” “De Quervain Disease, ” “Tendi- nopathy,”and “Tenosynovitis.” The terms were combined using Boolean search syntax to construct the search algorithm: ((“Lasers”OR “Laser Therapy”OR “Phototherapy”OR “Low-Level Light Therapy” OR “Laser Class IIIb”) AND (“Musculoskeletal Pain” OR “De Quervain Disease” OR “Tendinop- athy” OR “Tenosynovitis”)).
Data extraction
Three researchers used the Rayyan web tool for the initial screening of article titles and abstracts across multiple databases during the data extraction phase [22], with searches for each database retrieved in nbib, ris, or ciw formats. The three researchers independently evaluated the selected studies and transferred the data to a standardised Microsoft Excel form. Titles and abstracts of potential RCTs or non- RCTs were carefully examined for inclusion. Data extracted from the included reviews encompassed author details, country of origin, publication year, participants, number of groups, selection criteria, treatments, relevant outcomes, assessment instances, conclusions, and funding sources.
Risk of bias and methodological quality
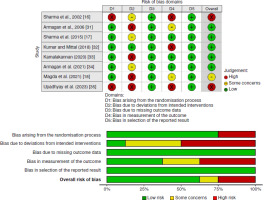
The methodological quality of RCTs was initially assessed using the PEDro scale [23]. The Cochrane Collaboration’s Risk of Bias 2 (RoB2) tool was applied to analyse bias across six domains within the included studies [24]: (a) bias arising from the randomisation process; (b) bias due to deviations from the intended intervention; (c) bias due to missing outcome data; (d) bias in outcome measurement; (e) bias in the selection of the reported result; and (f) overall bias. Studies with a PEDro score of five or less or two or more high-risk bias assessments were categorised as low-quality [25]. The assessment of bias agreement among three researchers (AM, GH, and HDB) was measured using the kappa statistic [26].
Statistical analysis
Statistical analysis was conducted using Review Manager software (RevMan 5.4). Heterogeneity among the studies was evaluated using the chi-squared (x2) test and the I2 statistic. The study weight was calculated as the inverse of its variance, with the combined effect determined as the weighted average of individual effects. Heterogeneity was considered significant when the I2 coefficient was greater than 50%. [27, 28]. Depending on the level of heterogeneity detected in the meta-analyses, either the Mantel-Haenszel fixed-effect method or the DerSimonian and Laird random-effects method was employed to compute the pooled effect, expressed as weighted mean difference (WMD) or standardised mean difference (SMD), along with 95% confidence intervals (Cl), for the reported outcomes of interest. Publication bias was planned to be assessed using funnel plots and Egger’s test [29, 30]
An electronic search for randomised controlled trials (RCT s) examining LLLT intervention in QT was conducted using the Quality of evidence
The evaluation of evidence quality used the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) approach, considering parameters such as study limitations, inconsistency, indirectness, imprecision, and publication bias [27]: (a) study limitations arising from blinding, allocation deficiencies, or overestimation of treatment effects; (b) inconsistency determined by heterogeneity (I2 > 50%) in main outcomes; (c) indirectness stemming from significant deviations in treated individuals or when compared to less common interventions; (d) imprecision, entailing uncertainty characterised by broad confidence intervals extending across the line of no effect in the meta-analysis, along with the requirement of an optimal sample size for relevance (n > 400); (e) when there were fewer than three relevant studies, publication bias could occur.
Evidence levels were assigned, from high to very low, with an initial high-quality rating attributed to each level owing to the exclusive inclusion of RCTs. Factors affecting GRADE domains can lead to a decrease in evidence quality. The significance of the evidence was juxtaposed to ascertain alignment with minimally clinically important differences (MCID). Researchers used the GRADEpro GDT tool to compile a summary table of evidence (www.gradepro.org) to ensure a thorough synthesis.
Literature search
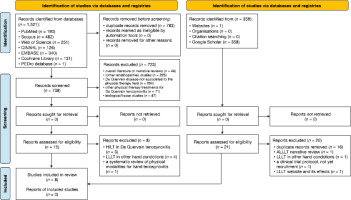
The search encompassed seven databases, yielding 1,319 articles as of the last update on March 10, 2025: PubMed (n = 190), Scopus (n = 482), WoS (n = 251), EBSCOhost (n = 126), Embase (n = 340), Cochrane Central (n = 131), and PEDro database (n = 1). Alternative methods, primarily manual searches on Google Scholar, identified 359 articles. After removing duplicates, 738 articles were selected for further analysis. Screening of titles and abstracts led to the preliminary selection of 15 articles, out of which eight were excluded: three studies on HILT in QT, four on LLLT in other hand con ditions, and one SR focusing on physical modalities for hand tenosynovitis. The alternative databases produced 21 documents, including 16 duplicates, with one study on LLLT in other hand conditions, one protocol study, and one webpage about the effects of LLLT. Appendix 1 presents an overview of the studies excluded from the databases, registries, and alternative methods, while Appendix 2 details the search strategy. Ultimately, eight RCTs were included in this review [16-18, SI- 35]. Figure 1 illustrates the search strategy using the PRISMA flowchart.
Quality assessment and risk of bias
The methodological quality assessment using the PEDro scale yielded an average score of 5.3 (± 1.3), with four studies rated as low quality (score < 6) (Table 1). The criteria with the lowest scores were therapist blinding (criterion 6), assessor blinding (criterion 7), and intention-to-treat analysis. In contrast, the highest scores were for adequate follow-up (criterion 8) and group comparisons at the end of treatment (criterion 11). Appendix 2 presents an evaluation of the PEDro scale.
Table 1
Study characteristics comparing LLLT for QT
| First author (year) country | PEDro score | Participants (n) mean age (± SD) | Inclusion criteria | Exclusion criteria | Groups (n) | Treatments | Sessions | Outcomes | Assessment instances | Results | Sources of funding |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sharma et al. (2002) [16] India | 3/10 | n = 28 ♂= 0; ♀ = 28) 33.3 ± 13.2 | - diagnosis of QT (uni or bilateral) | - cervical spondylosis with or without radiating pain - hypertension - diabetes - RA | EG (13) CG (15) | EG: LLLT Cg: placebo The use of drugs was prohibited during the study, | 3 and 10 s | (A) PI (VAS: Finkelstein test) (B) tenderness over radial styloid (Ritchie index) (C) grip and pinch strength (sphygmomanometer) (D) synovial sheath (USG) | TO: baseline T1 : post-treatment (2 weeks) | EG: l PI: 4 tenderness over radial styloid: 4 synovial sheath* and f grip and pinch strength* Cg: l PI: 4 tenderness over radial styloid: 4 synovial sheath* and f grip and pinch strength* Eg < CG: | PI: 4 tenderness over radial styloid and 4 synovial sheath* Eg > CG: f grip strength* | University College of Medical Sciences and Guru Teg Bahadur Hospital, Shahdara, Delhi |
| Armagan et al. (2006) [31] Turkey | 5/10 | n = 17 ♂ = NS; ♀ = NS) NS | NS | NS | EG (9) CG (8) | EG: LLLT + wrist splint Cg: placebo + wrist splint | 10 s | (A) PI (VAS) (B) Handgrip strength (sphygmomanometer) (C) global Improvement reported by the patient (VSGI) | TO: baseline T1 : post-treatment (10 days) | EG: ], Pi*: t VSGI* and | grip strength Cg: 4 Pi*: t VSGI* and t grip strength Eg < CG: 4 PI* Eg = CG: t VSGI and | grip strength | not reported |
| Sharma et al. (2015) [17] India | 6/10 | n = 30 ♂ = 2; ♀ = 28) 36.6 ±7.2 | - diagnosis of QT - without treatment at least (3 months) - Finkelstein test (+) | - cervical spondylosis with or without radiating pain - hypertension - diabetes - upper extremity fractures - RA | EG (15) CG (15) | EG: LLLT Cg: US | 7 s (2 weeks) | (A) PI (VAS) (B) tenderness over radial styloid (Ritchie index) (C) grip strength (sphygmomanometer) (D) synovial sheath (USG) | TO: baseline T1 : post-treatment (2 weeks) | EG: 4 PI*: 4 tenderness over radial styloid: 4 synovial sheath* and t grip strength* Cg: 4 PI*: 4 tenderness over radial styloid: 4 synovial sheath* and f grip strength* Eg = CG: 4 PI: 4 tenderness over radial styloid: 4 synovial sheath and f grip strength | not funded |
| Kumar and Mittal (2018) [32] India | 6/10 | n = 60 ♂ = 6; ♀ = 54) 35.4 ±6.9 | - age 25-35 years - positive Finkelstein test (+) | - cervical spondylosis - hypertension - diabetes - carpal tunnel syndrome - superficial radial neuritis - upper limb fractures - RA | EG (30) CG (30) | EG: LLLT + US CG: LCH injection | 7 s (3 weeks) | (A) PI (VAS) (B) tenderness over radial styloid (Ritchie index) (C) grip strength (sphygmomanometer) (D) synovial sheath (USG) | TO: baseline T1 : post-treatment (16 weeks) | EG: 4 PI*: 4 tenderness over radial styloid: 4 synovial sheath and | grip strength* Cg: 4 PI*: 4tenderness over radial styloid: 4 synovial sheath and f grip strength* Cg < GE: 4 PI* Eg > CG: f grip strength* Eg = CG: 4 synovial sheath | self-funding |
| Kamalakan- nan (2020) [33] India | 6/10 | n = 30 ♂ = NS; ♀ = NS) NS | - diagnosis of QT (uni or bilateral) - Finkelstein test (+) | NS | EG (15) CG (15) | EG: LLLTy K-Tape CG: US +TE | 6 s (2 weeks) | (A) PI (VAS) (B) PI affecting QoL (PRWE) | TO: baseline T1 : post-treatment (2 weeks) | EG: 4 Pi* and f pain affecting QoL Cg: 4 PI* and f pain affecting QoL Eg < CG: 4 PI* and pain affecting QoL | not reported |
| Armagan et al. (2021) [34] Turkey | 7/10 | n = 35 ♂ = o;♀ = 35) 42.7 ± 1.3 | - diagnosis of QT - pain and tenderness with palpation over the first extensor region - Finkelstein test (+) | - patients who had less pain in 30 days: - history of taking NAIDs - history of wrist fracture - concomitant disease: diabetes, Ra, gout, pseudo gout, pregnant or lactatlng mothers, and hypertension - corticosteroid Injection - surgery - abnormal findings (wrist radiography) - skin conditions making splint wear problematic | EG (18) CG (17) | EG: LLLT + wrist splint Cg: wrist splint | 15 s (3 weeks) | (A) PI (VAS) (B) handgrip strength (DNM) (C) global Improvement reported by the patient (VSGI) | TO: baseline T1 : post-treatment (3 weeks) | EG: 4 PI*: t handgrip strength* and | global Improvement reported by the patient* Eg: 4 PI: t handgrip strength and | global Improvement reported by the patient Eg = CG: 4 Pi: f handgrip strength and f global Improvement reported by the patient | not reported |
| Magda et al. (2021) [18] Egypt | 5/10 | n = 30 ♂ = 0:♀ = 30) 28.3 ±2.8 | - age 25-35 years -BMI <30 kg/m2 | - diabetes - cardiovascular deseases -OA | EG (15) CG (15) | EG: LLLT + TE Cg: TE | 12 s (4 weeks) | (A) PI (VAS) (B) cortisol levels (blood specimen) | TO: baseline T1 : post-treatment (4 weeks) | EG: 4 PI* and 4 cortisol levels* Cg: 4 PI* and 4 cortisol levels* Eg < CG: PI* and 4cortisol levels* | not reported |
| Upadhyay et al. (2023) [35] India | 4/10 | n = 90 ♂=19:♀ = 71) NS | - QT signs and symptoms with Usg confirmation - no history of previous treatment - VAS > 5 cm - Finkelstein test (+) | - history of trauma | EG (14) CG 1 (32) CG 2 (25) CG 3 (19) | EG: LLLT Cg 1: US Cg 2: corticosteroid Injection Cg 3: surgical release | 10 s (4 weeks) | (A) PI (VAS) (B) disability (DASH) (C) therapy satisfaction (self-report) | TO: baseline T1 : post-treatment (4 weeks) T2: follow-up (2 weeks after treatment) T3: follow-up (6 weeks after treatment) T4: follow-up (10 weeks after treatment) T5: follow-up (24 weeks after treatment) | EG: 4 PI*: 4 disability* and"f therapy satisfaction Cg 1: 4 PI*: 4 disability* and f therapy satisfaction Cg 2: 4 PI*: 4 disability* and f therapy satisfaction Cg 3: 4 PI*: 4 disability* and f therapy satisfaction Cg 4 < CG 3 < CG 2 < CG 1:4 PI* and 4 disability* | not funded |
[i] $- men, women, BMI - bone mass index, CG - control group, DASH - the disabilities of the arm: shoulder and hand questionnaire, DNM - dynamometry, EG - experimental group, LCH - Langerhans cell histiocytosis, LLLT - low-level laser therapy, OA- osteoarthritis, PI - pain intensity, PRWE - patient-rated wrist evaluation, QoL- quality of life, QT - de Quervain’s tenosynovitis, NAIDs - non-steroldal antiinflammatory drugs, NPRS - numeric pain rating score, NS - not specified, RA- rheumatoid arthritis, T1, T2..., TX - evaluations carried out after treatment, TENS - transcutaneous electrical nerve stimulation, US - therapeutic ultrasound, USG, ultrasonography, VAS - visual analog scale, VSGI - verbal scale global improvement; * p < 0.05 ias arising from the randomisation process Bias due to deviations from intended interventions Bias due to missing outcome data Bias in measurement of the outcome Bias in selection of the reported result
Figure 2 depicts the RoB evaluation with the RoB2 tool, administered by three researchers with a significant concordance (k = 0.76) [26]. The criteria with the highest RoB were deviations from intended interventions (50%) and bias in outcome measurement (37.5%). No bias was observed in “missing outcomes” or “bias due to selective reporting.” The overall bias was assessed as 25%.
Characteristics of the included studies
Table 1 summarises the characteristics of the included studies, specifying the author, country, number of participants, study groups, interventions, treatment sessions, outcomes assessed, and findings. The studies were conducted in India [16, 17, 32, 33, 35], Turkey [31,34], and Egypt [18] between 2002 and 2023. The total number of participants was 320, including 27 men and 246 women with an average age of 35.3 years (± 6.3). Two studies did not specify participant gender [31,35]. A total of 129 subjects received LLLT, either alone [16, 17, 35] or combined with other interventions such as splint immobilisation [31,34], therapeutic US [32], K-Tape [33], and therapeutic exercise [18]. The controls included 191 participants treated with wrist splints [31,34] and US [17, 33, 35]. Some controls were treated with corticosteroid injections, decompression surgery [35], and Langerhans Cell Histiocytosis (LCH) injections [32]. Only one study included a placebo control for LLLT [31].
Outcomes
Pain was assessed using tools such as the VAS [16-18, 32, 33-35] and the Ritchie Index for tenderness [16, 17]. Three studies evaluated grip strength using a sphygmomanometer or manual dynamometry [16, 17, 34]. Two studies employed USG to gauge the thickness of the synovial sheath within the initial compartment, measuring both anteroposterior and mediolateral diameters [16, 17]. Only one study evaluated disability using the DASH questionnaire [35]. Other outcomes assessed included patient-reported improvement using the Verbal Scale Global Improvement (VSGI) [31,34], quality of life using the Patient-Rated Wrist Evaluation (PRWE) [33], and blood cortisol levels [18].
LLLT dosage
Table 2 outlines the LLLT parameters used in the studies. All lasers were infrared As-Ga-Al diodes with wavelengths of 830 nm, except for one study that used 904 nm [33]. The mean treatment power ranged between 30 and 100 mW, with spot applications over the radial styloid process. Only one study reported a combination of punctual and scanning applications [17]. The spot size varied between 1.57, 4.9, and 12.6 cm2. Treatment time ranged from 100 to 600 s, with a mean of 284 s (± 200.6). Energy dosage ranged from 3 to 24 J, with a mean of 16.3 J (± 16.5).
Table 2
Characteristics and parameters of the lasers used in the included studies
| Characteristics/Parameters | Sharma et al. (2002) [16] | Armagan et al. (2006) [31] | Sharma et al. (2015) [17] | Kumar and Mittal (2018) [32] | Kamalakannan (2020) [33] | Armagan et al. (2021) [34] | Magda et al. (2021) [18] | Upadhyay et al. (2023) [35] |
|---|---|---|---|---|---|---|---|---|
| Laser model | endolaser 476 (enraf nonius) | endolaser 476 (enraf nonius) | endolaser 476 (enraf nonius) | endolaser 476 (enraf nonius) | NS | endolaser 476 (enraf nonius) | NS | NS |
| Wavelength (nm) | 830 nm(diode Ga-As-AI) | 830 nm(diode Ga-As-AI) | 830 nm(diode Ga-As-AI) | 830 nm(diode Ga-As-AI) | 904 nm(NS) | 830 nm(diode Ga-As-AI) | 830 nm(diode Ga-As-AI) | NS |
| Mode (continuous/pulse) | continuous | continuous | continuous | NS | NS | continuous | continuous | NS |
| Output power (mW) | 30-40 mW | 100 mW | 30-40 mW | 30-40 mW | NS | 100 mW | 30-40 mW | NS |
| Mean power (mW) | 30-40 mW | 100 mW | 30-40 mW | NS | NS | 100 mW | 30-40 mW | NS |
| Frequency (Hz) | NS | NS | NS | |||||
| Phase duration (ps) | NS | NS | NS | |||||
| Spot size (cm2) | 4.9 cm2 | 1.57 cm2 | 4.9 cm2 | NS | NS | 1.57 cm2 | 12.6 cm2 | NS |
| Treatment protocol | punctual on radial styloid | painful dorsa Radial area | punctual and scanning along radial styloid | punctual on radial styloid | punctual on radial styloid | painful dorsa Radial area | painful dorsa radial area (10 tender points) | NS |
| Energy density (J/cm2) | 4 J/cm2 or 2 J/cm2 (according PI with VAS) | NS | 3 J/cm2 or 2 J/cm2 (according PI with VAS) | NS | 0,25 y 1,3 J/cm2 | NS | 20 J/cm2 | NS |
| Total energy (J) | NS | 3 J | NS | 37 J | NS | 3 J | 18-24 J | NS |
| Treatment area (cm2) | 4 cm2 | NS | 3 cm2 | NS | NS | NS | NS | NS |
| Treatment time (s) | NS | 300 s | 100 s | 120 s | NS | 300 s | 600 s (60 s per point) | NS |
Results
Table 3 summarises intragroup results for the outcomes of interest. Both the experimental group (EG) and control group (CG) exhibited statistically significant disparities in pain intensity (p < 0.05) [16-18, 31-35], disability [35], and muscle strength [16, 17, 32, 34]] at the end of the treatment. Other outcomes, such as synovial sheath thickness of the first compartment [16, 17], blood cortisol levels [18], and quality of life [33], also showed marked changes in both groups. Three studies did not provide sufficient quantitative information for the reported outcomes in terms of central tendency and dispersion measures [16, 31,32].
Table 3
Results and Intragroup statistical comparisons for outcome measures before and after treatment
| Author | Outcome (instrument) | EG | CG | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline mean ± SD | post-treatment mean ± SD | p-value | efficacy | Baseline mean ± SD | post-treatment mean ± SD | p-value | efficacy | ||
| Sharma et al. (2002) [16] | PI (VAS - Finkelstein test) | Without quantitative data | |||||||
| tenderness over radial styloid (Ritchie index) | |||||||||
| grip strength (mm Hg) | 130.0 ± 35 | 150.0 ± 45 | < 0.01* | Yes | 125.0 ± 35 | 110.0 ± 20 | < 0.01* | Yes | |
| pinch strength (mm Hg) | 60.0 ± 10 | 70.0 ± 10 | 62.0 ± 12 | 55.0 ± 15 | |||||
| synovial sheath - AP (USG, cm) | 0.63 ± 0.21 | 0.52 ± 0.41 | 0.65 ± 0.1 | 0.72 ± 0.8 | |||||
| synovial sheath - ML (USG, cm) | 0.60 ± 0.3 | 0.49 ± 0.6 | 0.60 ± 0.2 | 0.64 ± 0.4 | |||||
| Armagan et al. (2006) [31] | PI at rest (VAS) | Without sufficient quantitative data | Without sufficient quantitative data | ||||||
| handgrip strength (sphygmomanometer) | |||||||||
| global improvement reported by the patient (VSGI) | |||||||||
| Sharma et al. (2015) [17] | PI at rest (VAS) | 8.8 ± 1.7 | 3.4 ± 3.5 | < 0.01 | 9.1 ± 1.5 | 4.2 ± 3.0 | < 0.01 | yes | |
| tenderness over radial styloid (Ritchie index) | without sufficient quantitative data | without sufficient quantitative data | |||||||
| grip strength (mm Hg) | 112.1 ± 42.8 | 133.9 ± 49.8 | < 0.01* | Yes | 117.6 ± 61.0 | 153.6 ± 70.8 | < 0.01* | Yes | |
| synovial sheath - AP (USG, cm) | 0.74 ± 0.14 | 0.9 ± 0.13 | 0.9 ± 0.13 | 0.9 ± 0.14 | |||||
| synovial sheath - ML (USG, cm) | 0.44 ± 0.13 | 0.4 ± 0.14 | 0.43 ± 0.01 | 0.41 ± 0.07 | |||||
| Kumar And Mittal (2018) [32] | PI (VAS - Finkelstein test) | 8.8 ± 1.6 | 4.2 ± 3.4 | < 0.01* | 9.1 ± 1.6 | 4.2 ± 3.4 | < 0.01* | yes | |
| tenderness over radial styloid (Ritchie index) | without sufficient quantitative data | ||||||||
| grip strength (sphygmomanometer, mm Hg) | 111.2 ± 42.4 | 136.4 ± 49.6 | < 0.01* | yes | 113.6 ± 49.6 | 196.32 ± 69.8 | < 0.05* | yes | |
| synovial sheath - AP (USG, cm) | Without sufficient quantitative data | Without sufficient quantitative data | |||||||
| synovial sheath - ML (USG, cm) | |||||||||
| Kamalakannan Et al. (2020) [33] | PI at rest (VAS) | 7.9 ± 0.7 | 4.1 ± 0.8 | < 0.01* | Yes | 8.0 ± 0.7 | 5.7 ± 0.6 | < 0.01* | Yes |
| PI affecting QoL (PRWE) | 8.1 ± 0.8 | 5.6 ± 0.8 | 8.1 ± 0.8 | 7.4 ± 0.3 | |||||
| Armagan et al. (2021) [34] | PI at rest (VAS) | 7.2 ± 1.6 | 6.0 ± 0.2 | < 0.01* | yes | 7.2 ± 1.6 | 7.1 ± 0.2 | < 0.01* | yes |
| grip strength (N) | 0.4 ± 0.01 | 0.4 ± 0.01 | 0.02* | 0.4 ± 0.01 | 0.4 ± 0.01 | 0.456 | no | ||
| global improvement reported by the patient (VSGI) | 3.4 ± 0.17 | 2.78 ± 0.14 | < 0.01* | yes | 3.06 ± 0.18 | 2.9 ± 0.15 | 0.379 | no | |
| Magda et al. (2021) [18] | PI at rest (VAS) | 3.6 ± 0.5 | 0.3 ± 0.5 | < 0.01* | Yes | 3.7 ± 0.5 | 2.5 ± 0.9 | < 0.01* | Yes |
| cortisol levels (blood specimen) | 19.6 ± 2.9 | 6.8 ± 1.2 | 19.3 ± 2.6 | 16.1 ± 2.6 | |||||
| Upadhyay et al. (2023) [35] | PI at rest (VAS): EG vs CG1 | 6.6 ± 0.8 | 1.5 ± 1.3 | < 0.01* | Yes | 6.5 ± 0.7 | 1.1 ± 0.8 | < 0.01* | Yes |
| PI at rest (VAS): EG vs CG2 | 6.5 ± 0.8 | 0.9 ± 0.8 | |||||||
| PI at rest (VAS): EG vs CG4 | 6.8 ± 0.6 | 0.4 ± 0.5 | |||||||
| disability (DASH - %): EG vs CG1 | 67.9 ± 7.5 | 5.7 ± 7.6 | 65.2 ± 6.7 | 2.3 ± 4.9 | |||||
| disability (DASH - %): EG vs CG2 | 64.7 ± 9.1 | 1.8 ± 4.1 | |||||||
| disability (DASH - %): EG vs CG3 | 68.9 ± 5.2 | 0 | |||||||
[i] Eg - experimental group, CG - control group, PI - pain intensity, VAS - visual analog scale, AP - anteroposterior view, ML - medial-lateral view, USG - ultrasonography, VSGI - verbal scale global improvement, QoL - quality of life, PRWE - Patient Rated Wrist Evalaution, DASH - the disabilities of the arm, shoulder, and hand questionnaire; * statistical significance
Meta-analysis
Pain intensity: The meta-analysis used six studies to scrutinise the analgesic effects of LLLT in contrast to alternative treatments (Figure 3A) [17, 18, 32-35]. The Dersimonian- Laird random effects model assessed the WMD derived from the MD, with differences in favour of LLLT observed (WMD = -0.98; 95% Cl: -1.91, -0.04; p = 0.04; EG (n) = 107, CG (n) = 124) with substantial heterogeneity [27, 28]. Sensitivity analysis by excluding studies with the lowest methodological quality showed a greater analgesic effect (WMD = -1.50 cm; 95% Cl: -2.11, -0.88; p = 0.01; EG (n) = 93, CG (n) = 93), but heterogeneity remained (Figure 3D).
Figure 3
Forest plots for pain intensity (visual analogue scale) (3A), strength (3B), and synovial sheath thickness (ultrasonography) at the end of treatment (3C). Sensitivity analysis excludes studies with lower methodological quality to manage heterogeneity (3D)
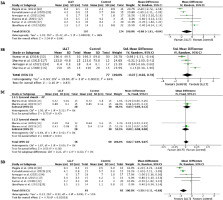
Figure 4 shows the evaluation of publication bias for pain intensity [29, 30]. The distribution for each mean difference (MD) measure extends from the middle of the funnel to the top, indicating good or fair precision given the homogeneous sample sizes, accompanied by a symmetrical MD distribution, although with values crossing the line of no effect. The Egg- er’s regression test ruled out publication bias (p = 0.161), suggesting the absence of a small study effect [30]. The evidence on the analgesic effects of LLLT was assessed as important, albeit with low certainty (Table 4).
Figure 4
Evaluation of publication bias for the pain intensity outcome using a funnel plot (left) and Egger's regression method (right)
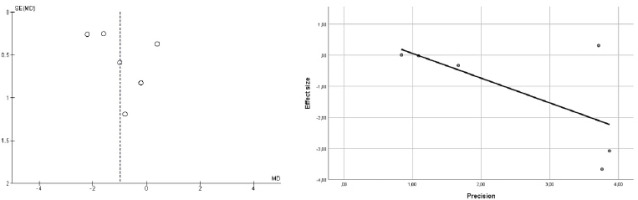
Table 4
Summary of findings and quality of evidence (GRADE) for the main outcome
[i] LLLT - low-level laser therapy, WMD - weighted mean difference, VAS - visual anolog scale (a) The overall risk of bias was generally low (25%). Sources of bias included outcome measurement (37.5%) and intervention deviations (50%); (b) The heterogeneity determines the inconsistency, depending on the I2 statistic £ 50%); (c) Considering a direct comparison of interventions and outcomes relevant to the study, with applicability to the clinical context, it was found that the indirect evidence held little significance; (d) Imprecision was assessed by examining the width of the confidence interval (Cl) for the pooled mean difference, the crossing of the no-effect line in the meta-analysis, and the sample size (n < 400); (e) The MD’s relationship to the minimally clinically important difference (MCID) determines the significance
Handgrip strength: The meta-analysis included four studies (Figure 3B) [16, 17, 32, 34]. The DerSimonian and Laird random effect analyses were applied to ascertain the SMD. No statistically significant differences favouring either group were discerned (SMD = -0.07; 95% Cl: -0.92, 0.78; p = 0.87; EG (n) = 76, CG (n) = 77), and substantial heterogeneity was present [27, 28]. The quality of the evidence was not assessed since there was no difference between the groups to warrant a recommendation grade.
Synovial sheath thickness: Two studies were included in the meta-analysis (Figure 3C) [16, 17]. Due to the absence of heterogeneity, the Mantel-Haenszel fixed-effect method was used. No differences were observed between the groups for the reduction in anteroposterior diameter (WMD = -0.07 mm; 95% Cl: -0.05, 0.12; p = 0.47; EG (n) = 28, CG (n) = 28) and mediolateral diameter (WMD = 0.00 mm; 95% Cl: -0.08, 0.08; p = 0.94; EG (n) = 28, CG (n) = 28) measured by USG. The quality of the evidence was not assessed since there was no difference between the groups to warrant a recommendation grade [29].
Disability: A meta-analysis was not conducted for disability because it was only reported as a relevant outcome by one study [35].
Discussion
Lllt is a non-thermal photobiological modality in the red or near-infrared spectrum used to stimulate tissue repair, reduce pain, and decrease inflammation. These effects have bolstered its use in managing various musculoskeletal conditions, including tendinopathies. With this background, the present review was developed to evaluate the analgesic efficacy of LLLT in QT treatment.
Although the main results show that LLLT can relieve pain, it is no more effective than control interventions at improving grip strength or reducing the thickness of swollen synovial sheaths. Despite the evident analgesic advantages, it is imperative to acknowledge the heterogeneity inherent in the meta-analysis, signifying variations across studies. Consequently, the assessment of the evidence is deemed significant, albeit with a degree of uncertainty.
LLLT and pain reduction
Lllt effectively reduces VAS-determined pain in QT patients when applied alone [16, 17, 35] and in combination with other physical therapy treatments such as splint immobilisation [31,34], US [32], K-Tape [33], and therapeutic exercise [18]. The results were significantly in favour of LLLT within and between groups. Nevertheless, these values fall below the reported minimal clinically important difference (MCID) of 1.37 cm for VAS [36]. Furthermore, it has been suggested that a change of 2.8 cm (± 2.1) for patients with baseline VAS pain scores of at least 6.7 can represent a clinically significant change in pain severity (MIC) from the patient’s perspective [37]. The meta-analysis did not reflect this MIC, but intragroup comparisons before and after LLLT treatment did.
Although LLLT is thought to have analgesic effects, particularly in nociceptive and neuropathic pain, the specific un derlying mechanisms are controversial. The widely accepted hypothesis suggests that the oxidised cytochrome c oxidase (CCO) enzyme, the terminal part of the electron transport chain, absorbs radiation because of its photosensitivity to light [11, 14]. CCO acts as a photoacceptor for red and infrared wavelengths through copper and iron chromophores, leading to increased cellular metabolism and ATP production. Additionally, LLLT promotes nitric oxide release through photodissociation from CCO, increasing the rate of ATP production and promoting vasodilation, thereby modulating reactive oxygen species (ROS) [38, 39]. ROS are implicated in inflammatory, neuropathic, and persistent pain, promoting neuronal excitability in nociceptive pathways and triggering mitochondrial dysfunction and neuroinflammation [38]. Additionally, ROS contribute to the activation of transient receptor potential channels (TRP), rendering transient receptor potential vanilloid 1 (TRPV1) more sensitive and activating transient receptor potential A1 (TRPA1) [40].
Lllt induces analgesia by stimulating endogenous opioid peptide synthesis, such as ß-endorphin, while simultaneously reducing the activity of bradykinin and C fibres [41]. Additionally, LLLT initiates morphological alterations in neurons, leading to a reduced mitochondrial membrane potential and a deceleration or inhibition of nerve conduction, thereby attenuating nociceptive transmission [10, 13, 41]. Neurons of the dorsal root ganglion (DRG) specifically exhibit these effects [42]. Furthermore, LLLT mitigates the release of pro-inflammatory neuropeptides, such as substance P and calcitonin gene-related peptide (CGRP), which play pivotal roles in neurogenic inflammation [39, 41].
Evidence suggests that LLLT may increase serotonin (5-HT) production in the central nervous system, favouring the activation of descending pain modulatory pathways [39, 41]. Through the 5-HT1a, 5-HT1b, 5-HT2c, 5-HT3, and 5-HT4 receptors, the 5-HT pathway from the rostral ventromedial medulla changes how pain signals are sent In the dorsal horn [43]. The diminution of signalling molecules Implicated In the Inflammatory cascade, including nuclear factor kappa B (NF-kB), which can hinder prostaglandin E2 (PGE2), tumour necrosis factor-alpha (TNF-a), cyclooxygenase-2 (COX-2), and interleukin 1 (IL-1), may also explain the analgesic effects of LLLT [39].
Heterogeneity observed for pain intensity
The I2 index determines heterogeneity, which was evident in the pain intensity meta-analysis. This heterogeneity indicated variability among the studies, which affects the validity of the conclusions drawn from combining the RCT results [27, 28]. These variations may be due to differences in sample size, randomisation, or the measurement of outcomes of interest [27, 28].
Despite attempts to perform a sensitivity analysis by excluding studies with lower methodological quality [35], significant heterogeneity persisted. Additionally, excluding studies can increase publication bias, leading to a small study effect that overestimates the results. Consequently, the authors opted to retain the initial analysis results.
LLLT and synovial sheath thickness
Lllt has been shown to effectively reduce synovial sheath thickness by the end of treatment, though it does not surpass control treatments such as US [17, 35]. This application is grounded in the anti-inflammatory effects of LLLT, supported by various physiological mechanisms. Indeed, wavelengths between 632 and 904 nm induce significant anti-inflammatory effects comparable to nonsteroidal anti-inflammatory drugs (NSAIDs) [44]. These effects include the inhibition and/ or attenuation of inflammatory mediators and pain markers, such as TNF-a, IL-ip, IL-6, COX-2, and PGE2 [44, 45].
In certain scenarios where corticosteroid injections are used to treat QT, the anti-inflammatory efficacy of LLLT may be compromised [35, 46]. Corticosteroid injections are successful in 73.4% of cases with two applications but have a higher failure rate in women with a high body mass index (BMI > 30 kg/m2) [46]. Therefore, LLLT could be an effective alternative to steroid injections in overweight women with QT.
There is evidence that both red and infrared LLLT have a dose-dependent anti-inflammatory effect, with a median average power output of 25 mW [44]. According to the ArndtSchultz law [47], biostimulation is observed at doses ranging from 0.05 to 10 J/cm2, with an optimal range between 0.5 and 4 J/cm2, which has been associated with reduced pain and inflammation [44, 48]. A dose of 0.6 J/cm2, with an irradiation time of at least 16 s, yields the lowest energy densities for anti-inflammatory effects, while a dose of 3 J/cm2 for 300 s yields better effects [44].
LLLT and grip strength
Although LLLT therapy focuses on pain reduction, this review demonstrates an increase in grip strength at the end of treatment, although it was not shown to be superior to control treatments. Pain influences the ability to generate muscle strength due to reflex inhibition and structural changes associated with the clinical condition [49, 50]. Moreover, a reduction in strength of up to 20-30% in a painful limb appears to be typical in chronic pain patients [51].
The assessment of grip strength is essential for understanding hand function. Therefore, the authors recommend maintaining it as a relevant outcome variable for future studies.
LLLT and disability
Despite the close relationship between pain and disability, only one study using the DASH questionnaire considered this variable as a relevant outcome [35]. Pain, particularly in chronic cases, commonly restricts functional capacity, leading to heightened pain perception due to physical inactivity and reduced mobility [52]. However, the relationship between the duration, intensity, extent, and meaning of pain does not always show a linear correlation with disability [52, 53]. The authors recognise the complexity of this construct and highly recommend the inclusion of functional outcomes, such as patient-reported outcome measures (PROMs) [54], in (RCTs) and clinical practice.
Recommendations
The optimal dose of As-Ga-Al LLLT to achieve analgesia remains controversial, and the World Association for Photobiomodulation Therapy (WALT) has not established specific dosage recommendations (https://waltpbm.org/). Based on the reviewed literature, a mean output power between 30 and 100 mW and an energy dose per point of 3 to 4 joules are suggested, with a minimum application of three points along the radius styloid.
Considering that conservative treatments have shown similar efficacy to injections and surgery, prioritising LLLT or non-thermal US before resorting to injections or surgery in cases of persistent pain is recommended. Using LLLT or US may prevent surgery-related complications such as damage to the superficial radial nerve or the entrapment of extensor tendons.
Although very few studies included therapeutic exercise in their treatments, it may be beneficial to add thumb extension isometric exercises and stretching alongside LLLT [55, 56].
Limitations
In this SR, the authors adhered to PRISMA guidelines [19], registered the protocol in PROSPERO [20], and conducted a comprehensive search across eight electronic sources [21]. However, two main limitations were identified:
The RoB may compromise the internal validity of the study due to deviations in the intended interventions and outcome measurement.
Heterogeneity among studies limits the quality and recommendation of the evidence. This variability is attributed to the limited number of RCTs available, which also precluded a sensitivity analysis to control for heterogeneity.
These limitations emphasise the need to conduct new RCTs on LLLT in QT with improved methodological quality.
Conclusions
Lllt emerges as a promising resource for the management of QT-associated pain. Although LLLT has demonstrated effectiveness in reducing pain, its benefits on manual grip strength and inflammation of the synovial sheaths do not appear to surpass treatments such as US, splint immobilisation, or corticosteroid injection. Despite acknowledging the analgesic effects of LLLT, the heterogeneity among studies and the low certainty of evidence underscores the need for future RCTs with better methodological quality. Given the close relationship between pain and functional limitation, future studies should maintain grip strength assessment as a relevant outcome measure and incorporate disability evaluation. Furthermore, the authors recommend prioritising the use of LLLT or US with strengthening and stretching exercises before resorting to invasive options such as injections or release surgery. These recommendations could enhance clinical management and minimise the risk of postsurgical complications.