Introduction
Low back pain (LBP) is a major cause of morbidity and affects approximately 80–85% of individuals in their lifetime [1]. The National institutes of Health (NiH) Task Force on Research Standards for Chronic LBP (CLBP) defines CLBP as pain that has continued for > 3 months [2]. it is further divided into two categories: specific (known cause) and nonspecific (unknown cause). European guidelines describe nonspecific CLBP as LBP whose pathological causes are unknown, such as osteoporosis, fracture, inflammatory diseases, and infection [3]. Approximately 80% of patients with LBP are diagnosed with nonspecific CLBP [4, 5].
Various treatment options are available for managing LBP. in addition to surgical and medical treatments, clinical guidelines recommend non-pharmacological and nonsurgical management of LBP, including physiotherapy [6, 7]. Therefore, various interventions, including manual, thermal, and aquatic therapies, have been used to manage CLBP [8–10].
Thermal therapy is widely used by both clinicians and patients as a management strategy for CLBP [11]. it is an umbrella term that indicates the different modalities that supply superficial or deep heating via conduction, convection, or conversion mechanisms. Hydrocollator packs and low-level heat wrap; fluid therapy and hydrotherapy; and heat lamp, ultra-sound, and diathermy are a few examples of conduction, convection, and conversion-based modalities, respectively [12]. diathermy uses a controllable frequency of electromagnetic waves to provide heat to tissues. Clinicians treating musculo-skeletal disorders, such as CLBP, widely use a diathermy approach called capacitive and resistive electric transfer (CRET) therapy [13, 14]. CRET uses approximately 0.5 MHz long-wave radiofrequency (RF) to supply heat in two therapeutic modes, namely capacitive electrode transfer (CET) and resistive electrode transfer (RET) modes, which are used for superficial and deep heating, respectively [15]. it is considered a safe form of diathermy with a low risk of skin burns [16].
Previous systematic reviews on the effects of CRET have examined a broad range of musculoskeletal disorders [17–19]. However, no published reviews have explicitly examined the effects of CRET on nonspecific CLBP. Given this knowledge gap, a comprehensive review of these promising interventions is required. Therefore, herein, we systematically reviewed and summarised the available evidence on the application of CRET in patients with nonspecific CLBP.
Subjects and methods
Research question
This study’s research question adheres to the Patient, intervention, Comparison, and outcome Study (PiCoS) model [20] and simultaneously abides by the Preferred Reporting items for Systematic Reviews and Meta-Analyses (PRiSMA) review guidelines as follows. Population: Patients aged 18–70 suffering from nonspecific CLBP that lasts longer than 3 months. intervention: deep thermotherapy using a RF device. Comparison: Any other type of treatment. outcome: Pain relief and improved quality of life.
Literature search
In the first stage, a set of keywords was designed to classify the concept into four categories: LBP, thermotherapy, physical therapy, and pain relief. These four categories belong to the PiCoS-based research questions defined in the preceding section. Table 1 lists the categories and corresponding keywords.
Table 1
Keywords and literature search strategies
In the second stage, a comprehensive search was performed using six electronic databases: PubMed, EMBASE, PEdro, MEdLiNE, CiNAHL, and Scopus. The keywords were combined using the Boolean command “oR,” and the categories were linked using the Boolean command “ANd”. A language and date filter were applied to exclude non-English studies and to limit the results to those between January 2000 and September 2023. Moreover, only studies published in full articles (not in abstracts or conference proceedings) were included. Google Scholar was used as a complementary search engine to ensure the inclusion of the maximum number of relevant studies. databases, such as PubMed, Embase, and MEdLiNE, have filtering options for limiting the search to “randomised controlled trials,” which was used when available to narrow down literature retrieval to randomised controlled studies.
Selection of studies
Eligible studies adhered to the criteria set in the “research question” subsection, that is, studies that included patients diagnosed with nonspecific CLBP (symptomatic for > 3 months and aged 18 and 70 years). No filter was applied to sex; however, studies dealing with specific CLBP were excluded. Further, no restriction was set for CRET being used alone or in conjunction with another form of treatment.
Two researchers (Si and KN) independently screened the titles and abstracts of the studies. if eligibility was not clear, the full text was screened, and the rationale for inclusion or exclusion was clarified. Any discrepancies were resolved through discussion with a third researcher (HU). Finally, the full texts were downloaded for all the articles that met the inclusion criteria for data extraction and analysis.
Data extraction
Selected attributes, including demographic characteristics, intervention device, frequency applied, intervention time frame, number of sessions, control group characteristics, outcome measures, and main results, were extracted by Si and KN independently from the articles that met the inclusion criteria. All attributes were tabulated in an Excel spreadsheet.
Risk-of-bias analysis
The articles included in this systematic review were assessed for the risk of bias according to the Cochrane guidelines [21]. The Cochrane tool for assessing the risk of bias is based on seven principles focusing on internal validity and excluding the use of quality scales. Selection, performance, detection, attrition, reporting, and others are six domains of bias covered under risk of bias assessment guidelines, and within each domain, one or more sources of bias are assessed.
Results
Literature search and study selection
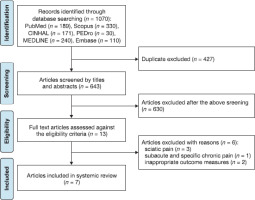
The database search initially resulted in 1070 relevant articles. duplicate data were obtained from several databases. Thus, duplicates were removed, and the remaining articles were screened against the selection criteria. Endnote 20 was used to manage the literature and remove duplicates. Finally, seven articles met the inclusion criteria and were included in this systematic review. of the seven studies, three were conducted in Japan [13, 22, 23] and two in italy [24, 25]. one study each was conducted in South Korea [26] and Greece [27]. Figure 1 illustrates the technical flow of this systematic review.
Characteristics of the included studies
Table 2 provides details about the main characteristics of all included trials. A total of 351 individuals participated in the selected studies. The study sample size ranged from 24–118, and the age range of participants, as reported in the selected studies, was 18–70 years, except for the study by Wachi et al. [23], which did not mention the ages of the participants. All included studies focused on nonspecific CLBP. Two studies compared CRET therapy with sham CRET therapy [22, 23]. other studies compared CRET in conjunction with exercise versus exercise only [13], deep heat therapy versus superficial heat [24], CRET plus manual therapy and manual therapy versus general instruction and counselling [27], and CRET compared with the alternative mode of CRET, capacitive followed by resistive [25].
Table 2
Characteristics of the included studies
| Study | Population | Total number of partici-pants | inter-vention group | Age of the participants (years) | intervention/ device | Frequency | Time | Number of sessions/ frequencies | Control group | outcome measures | Main results | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [13] | NSCLBP | 30 | 14 | 20–50 | CRET by iNdiBA Active Pro Recovery HCR 902 plus exercise | 448 kHz | 15 min | 10 (2 or 3/week) | Exercise Only | VAS, odi, and modified Kraus–Weber test | Reduced pain and improvement in functional disability in the ‘CRET plus exercise group’ as compared to the control group | |
| [23] | NSCLBP | 24 | 12 | iG: 34.3± 8.7 CG:32.5 ± 7.5 | CRET using Physio Radio Stim Pro | 500 kHz | 15 min | 1 session only | Sham CRET | VAS, EMG, and US | Improvement in LBP and muscle stiffness in the CRET group compared with the sham group | |
| [22] | NSCLBP | 30 | 15 | iG: 35.5 ± 9.4 CG: 34.8 ± 10.1 | CRET using Physio Radio Stim Pro | 500 kHz | 15 min | 1 session only | Sham CRET | VAS and EMG | Decreased pain and improved muscle activity in the CRET group compared with the sham group | |
| [26] | NSCLBP | 118 | 62 | iG: 46.5 ± 14.0 CG: 48.9 ± 12.5 | Radiofrequency using HiPER-500, RF-diathermy | 4.4 MHz | 10–15 min | 12 (3/week) | Therapeutic ultrasound | NPRS, odi, up-and-go test, Biering– Sorensen test, and NASS | The RF group showed greater improvement in outcome measures in the study, including pain and back muscle endurance, compared to the ultrasound group | |
| [27] | NSCLBP | 60 | 40 | iG(i): 37.85 ± 2.62 iG(ii): 39.20 ± 2.63 CG: 38.10 ± 2.36 | one group: manual therapy Second group: TECAR plus manual therapy WinBack-TECAR device | 500 kHz | 30 min | 6 (3/week) | Received instruction and counselling | NPRS, RMdQ, PPT, and FTF | The MT plus TECAR group showed greater improvement in pain, disability, and PPT compared with manual therapy and control groups. | |
| [24] | NSCLBP | 49 | 24 | iG: 60.6 ± 10.4 CG: 59.4 ± 12.0 | deep heating therapy using the dHT-Emaildue device | 450 kHz | 20 min | 10 sessions in 15 days | Superficial heating | NPRS and odi | Reduced pain and disability in the deep heating group as compared with the superficial heating group | |
| [25] | NSCLBP | 40 | 20 | 23.2 ± 2.5 | Resistive mode followed by capacitive mode using the Quilmed-Endor device | 500 kHz | 20 min | 1 session only | Capacitive mode followed by resistive mode | digital pressure algometry and thermal imaging | TECAR therapy improved pain and increased the tissue temperature in both groups | |
| NSCLBP – nonspecific chronic low back pain | NPRS – Numerical Pain Rating Scale, | |||||||||||
| LBP – low back pain | NASS – North American Spine Society | |||||||||||
| CRET – capacitive and resistive electric transfer | PPT – pressure pain threshold | |||||||||||
| VAS – visual analogue scale, | RMdQ – Roland–Morris disability Questionnaire, | |||||||||||
| odi – oswestry disability index | FTF – lumbopelvic region mobility with the fingertip-to-floor | |||||||||||
| EMG – electromyography | iG – intervention group | |||||||||||
| US – ultrasonography | CG – control group | |||||||||||
Device protocol
Of the seven studies, only two used a Physio Radio Stim Pro device (SAKAi Medical Co., Ltd., Tokyo, Japan) [22, 23]. one study used iNdiBA Active Pro recovery HCR 902 (iNdiBA S.A., Barcelona, Spain) [13], whereas another reported the use of a HiPER-500 RF-diathermy apparatus (JS-oN Corporation, Seoul, South Korea) [26]. WinBack-TECAR (WiNBACK 3SE, Villeneuve Loubet, France) [27], dHT-Emaildue (Ferrara, italy) [24], and Quilmed-Endor (Neurolinks Srl, Rome, italy) [25] were used in the remaining three studies. The frequency range reported in the six studies was 448–500 kHz, whereas one study used a frequency of 4.4 MHz. All included studies explained the treatment protocol in detail. in all the studies, the position of the participant and electrode placement were well explained. They applied an RF current to the lower lumbar region. in three of the seven studies, the RF was initially applied in the capacitive mode (5 min) followed by the resistive mode (10 min) [13, 22, 23]. The average duration of intervention in all the included studies was 18.57 min. Tashiro et al. [13], Lee et al. [26], Kasimis et al. [27], and Zati et al. [24] also performed follow-up treatments, whereas three studies [22, 23, 25] provided one treatment session and mainly focused on checking the immediate effects of the intervention. in the follow-up studies, measurements were taken before and immediately after the intervention and after 2, 4, and 12 weeks.
Outcome index
Overall, the visual analogue scale (VAS), numerical pain rating scale (NPRS), and pain pressure threshold (PPT) were used in the studies as pain measurements. Two studies quantified pain, muscle stiffness, and muscle activity using electro-myography and ultrasonography [22, 23]. one study used the VAS, oswestry disability index (odi), and modified Kraus– Weber test to measure pain, functional disability status, and abdominal and psoas muscle strength [13]. one study measured pain and functional disability status, back extensor muscle strength, gait speed, balance, and patient satisfaction using the NPRS, odi, up-and-go test, Biering-Sorensen test, and North American Spine Society guidelines [26]. Three studies measured pain, functional disability status, pressure pain threshold, tissue temperature, and lumbar spine range of motion using the NPRS, digital algometry, odi, Roland-Morris disability Questionnaire, thermal imaging, and lumbopelvic area movement using the fingertip-to-floor test [24, 25, 27].
Synthesis of results
Two studies by Wachi et al. [22, 23] compared CRET with sham CRET. The treatment group showed improvements in pain and muscle stiffness and facilitated muscle activity, which improved their quality of life. one of the seven studies compared 4.4-MHz RF with ultrasound therapy. According to the outcomes of that study, the RF diathermy group had greater improvements in pain, function, mobility, and back muscle endurance compared to the ultrasound group [26]. one of the seven studies compared CRET plus exercise to exercise only. The CRET plus exercise group outperformed the exercise-only group in pain and functional disability outcomes [13].
A study compared CRET in combination with manual therapy (group A) to manual therapy alone (group B) and a control group (group C). Group A showed greater improvement in pain, disability, and PPT compared with groups B and C [27]. Moreover, Zati et al. [24] compared deep heat therapy to superficial heat therapy and reported that the deep heat therapy group showed greater improvements in pain and disability compared to the superficial heat group. Finally, Barassi et al. [25] used CRET in both groups; however, the order of treatment was different: resistive mode followed by capacitive mode and vice versa. Their findings showed that CRET therapy improved pain and increased tissue temperatures in both groups.
Quality assessment of studies
PEdro scale scores were used to assess the quality of the included trials. The scores were downloaded from the PEdro database (https://pedro.org.au). if a study lacked a PEdro rating, two skilled PEdro raters who had undergone the PEdro scale rating tutorial assigned scores to the study.
The PEdro scale consists of 11 items, 10 of which are used to calculate scores that provide information about the internal validity of the trials and the reported statistical information. Each item in each trial is scored out of 10, with higher scores indicating greater reliability and validity [28, 29].
The scores for two out of the seven trials were available in the PEdro database [24, 26]. The other five trials [13, 22, 23, 25, 27] were scored by two experts (Table 3). The mean score of all included studies was 6.43. in the selected trials, blinding of the patients, therapists, and accessors was not possible because of the complex combination of interventions. There is no published validated cutoff score for this scale. Therefore, trials with a score of < 5 points are considered low-quality, and trials with a score of > 5 points are considered high-quality trials [29]. Six of the seven trials had scores > 5, and one had a score of < 5.
Table 3
PEdro scale for the included studies
| Study | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|
| [13] | – | + | + | – | – | – | + | + | + | + | 6/10 |
| [23] | + | + | + | – | – | – | – | + | + | + | 6/10 |
| [22] | + | + | + | + | – | – | – | + | + | + | 7/10 |
| [27] | + | + | + | – | – | – | + | + | + | + | 7/10 |
| [26] | + | + | + | + | – | + | + | + | + | + | 9/10 |
| [25] | + | + | – | + | – | – | – | + | + | + | 6/10 |
| [24] | + | – | + | – | – | – | – | – | + | + | 4/10 |
[i] 1 – randomisation, 2 – allocation concealment, 3 – comparability at baseline, 4 – patient blinding, 5 – therapist blinding, 6 – assessor blinding, 7 – at least 85% follow-up, 8 – intention-to-treat analysis, 9 – between-group statistical comparisons, 10 – point measures and measures of variability (+) – item fulfilled, (–) – item not fulfilled
Risk of bias of randomised controlled trials
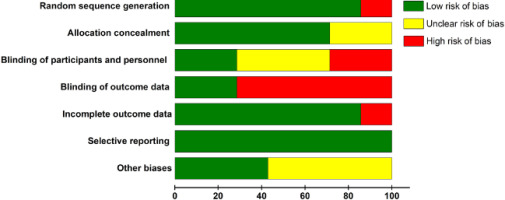
The included studies were analysed for the risk of bias using the Cochrane tool for assessing the risk of bias (explained in Section 2.5). Each study underwent evaluation based on the seven bias sources (Figure 2). Low, unclear, and high risks (of bias) are presented in green, yellow, and red, respectively. overall, a low risk of bias (green colour) was dominant in all sources, except for in “blinding of outcome data.” All studies exhibited a low risk of bias in “selective reporting.” Regarding “random sequence generation” and “incomplete outcome data,” 87.5% of the articles had a low risk, whereas the remaining articles exhibited a high risk of bias. Regarding “blinding of participants and personnel,” 37.5% of the studies had a low risk of bias, 37.5% had an unclear risk, and approximately 25% had a high risk of bias. Regarding “blinding of outcome data,” 25% of the articles had a low risk of bias, 12.5% had unclear bias, and the remaining 62.5% had a high risk of bias. in “allocation concealment,” approximately 75% of studies were rated as low risk, whereas the remaining 25% of the articles had unclear risk of bias. Regarding “other biases,” an equal number of studies showed low and unclear risk of bias, with no study exhibiting a high risk of bias.
Discussion
This systematic review aimed to synthesise the effects of CRET on outcomes, including pain reduction and improved quality of life, in patients with nonspecific CLBP. our review identified seven studies (randomised controlled trials) using a systematically devised search strategy, and relevant attributes were extracted and analysed to understand the effectiveness of CRET on nonspecific CLBP. in addition, the risk of bias was analysed using the Cochrane tool. in all included trials, the treatment protocols were clearly defined, in addition to stating the frequency range of CRET therapy and the duration of the intervention. in all trials, CRET was compared with standard care (exercise or ultrasonography) or sham CRET (CRET power off). Furthermore, in this type of study, it was not feasible to blind therapists because the electrodes and the patient’s skin may become heated during treatment. in addition, even in the case of sham CRET, patients can feel the absence of heat on the skin. This may psychologically affect treatment outcomes.
The results further demonstrate that CRET is an effective option for immediate and long-term follow-up treatment for pain reduction and improved physical function among patients with nonspecific CLBP. The findings of the included articles showed improvement in pain and enhanced muscle elasticity, muscle activity, and physical function. Particularly, studies with a 1–3-month follow-up treatment design showed significant progress in pain reduction and enhanced physical function at the conclusion of each follow-up. For instance, in a 1-month follow-up study conducted by Tashiro et al. [13] using 448kHz RF, CRET plus exercise showed more improvement in pain intensity compared to the exercise alone group (p < 0.05). Studies with only one CRET treatment session also showed a significant reduction in pain, enhanced muscle elasticity, and improved muscle activity in post-treatment measurements. Zati et al. [24] showed a significantly better outcome in terms of disability using deep heating CRET (450 kHz) compared to superficial heating therapy (p = 0.015) by comparing the baseline data to that recorded 15 days later. overall, these studies demonstrate that CRET is effective alone and in combination with other physical modalities or manual therapy for pain improvement.
Studies using CRET for nonspecific CLBP are limited; evidence from RF usage on other forms of musculoskeletal pain can be used to augment the findings of this review. For instance, Paolucci et al. [30] applied CRET to patients with painful shoulder impingement syndrome and compared the results with sham CRET. The findings indicated that the group that received CRET therapy showed an improvement in pain. Beltrame et al. [19] conducted a review of 13 studies that used CRET to treat musculoskeletal pain. Their findings revealed that almost all 13 studies reported lower pain levels and improvement in muscle properties. CRET is a type of diathermy that follows the principle of superficial or deep tissue heating at a certain frequency. The heat from CRET results in increased blood circulation, decreased pain, enhanced muscle recovery, and increased muscle flexibility [16]. our review has a few potential limitations. First, it only included English-language articles. This implies that there may be additional evidence on the effectiveness of RF for nonspecific CLBP published in other languages. Second, we excluded articles that applied CRET to specific CLBP because it was outside the scope of this review. A separate review is warranted to understand the effects of CRET on specific CLBP. Finally, this review was not registered in the international Prospective Register of Systematic Reviews.
Conclusions
This is the first systematic review of the effectiveness of CRET on nonspecific CLBP. CRET is a deep thermotherapy technique that is the most popular and widely used in clinical settings. it is a safe form of diathermy with a negligible risk of skin burns. This systematic review delivered a detailed synthesis of the scientific literature on the use of CRET for non-specific CLBP. The results show that CRET is an effective deep-heating modality for reducing pain, decreasing disability, and improving the quality of life in patients with nonspecific CLBP. The pathological explanation of the effectiveness of CRET includes vasodilation due to heating and a resulting increase in haemoglobin saturation. it is expected that more randomised controlled trials will be conducted in the future to strengthen the evidence on the effectiveness of CRET on nonspecific CLBP.